Abstract
Background and Purpose
Excessive daytime sleepiness and sudden sleep attacks are the main features of narcolepsy, but rapid-eye-movement sleep behavior disorder (RBD), hyposmia, and depression can also occur. The latter symptoms are nonmotor features in idiopathic Parkinson's disease (IPD). In the present study, IPD-proven diagnostic tools were tested to determine whether they are also applicable in the assessment of narcolepsy.
Methods
This was a case-control study comparing 15 patients with narcolepsy (PN) and 15 control subjects (CS) using the Scales for Outcomes in Parkinson's Autonomic Test (SCOPA-AUT), Parkinson's Disease Nonmotor Symptoms (PDNMS), University of Pennsylvania Smell Test, Farnsworth-Munsell 100 Hue test, Beck Depression Inventory, and the RBD screening questionnaire.
Results
Both the PN and CS exhibited mild hyposmia and no deficits in visual tests. Frequent dysautonomia in all domains except sexuality was found for the PN. The total SCOPA-AUT score was higher for the PN (18.47±10.08, mean±SD) than for the CS (4.40±3.09), as was the PDNMS score (10.53±4.78 and 1.80±2.31, respectively). RBD was present in 87% of the PN and 0% of the CS. The PN were more depressed than the CS. The differences between the PN and CS for all of these variables were statistically significant (all p<0.05).
Conclusions
The results of this study provide evidence for the presence of dysautonomia and confirm the comorbidities of depression and RBD in narcolepsy patients. The spectrum, which is comparable to the nonmotor complex in IPD, suggests wide-ranging, clinically detectable dysfunction beyond the narcoleptic core syndrome.
Narcolepsy is a sleep-wake disorder characterized by excessive daytime sleepiness (EDS) with sudden onset and an abnormal transition from wakefulness into rapid eye movement (REM) sleep. Other typical features are cataplexy, hypnagogic hallucinations, sleep paralysis, and automatic behavior. The International Classification of Sleep Disorders defines two subtypes of this disorder: narcolepsy with and without cataplexy. It is currently thought that narcolepsy is attributable to autoimmune processes that trigger a specific cell loss in the lateral and posterior hypothalamus,1 but more rarely it can be of symptomatic origin (e.g., inherited disorders, tumors, or multiple sclerosis).2 Hypothalamic cells secrete hypocretin and are integrated into different CNS networks (i.e., the reticular activating system, and the olfactory and autonomic systems). Although sleep-wake dysfunction is the cardinal syndrome, other symptoms may also occur: REM sleep behavior disorder (RBD), hyposmia, depression, and obesity. Clinical observations suggest that several of the encountered symptoms can also be seen in patients suffering from idiopathic Parkinson's disease (IPD). Interestingly, RBD and hyposmia are often proposed as harbingers of IPD.3,4 We hypothesize that further commonalities exist between narcolepsy and IPD.
Using validated and well-introduced IPD evaluation questionnaires and clinical tests, an exploratory case-control study was conducted to determine whether other nonmotor symptoms that are routinely found in IPD also occur in patients with narcolepsy (PN). In particular, we explored whether PN exhibit autonomic dysfunction or sensory deficits. However, since it was not possible to directly compare PN with IPD patients, they were compared directly with age-matched healthy control subjects (CS), and the observed scores were post-hoc compared with those reported in the literature for IPD patients, while remaining mindful of the methodological limitations of such an approach.
Fifteen consecutively seen PN were prospectively enrolled in this study and were compared with 15 volunteer CS who were matched for gender and age. Narcolepsy with or without cataplexy was diagnosed using the International Classification of Sleep Disorders II criteria, and polysomnography and the multiple sleep latency test. Hypocretin concentration in the cerebrospinal fluid (CSF) was not assessed since hypocretin status is not required for a clinical diagnosis. The following PN exclusion criteria were applied: any Parkinsonian features, secondary narcolepsy, and any preexisting diseases with autonomic dysfunction (e.g., gastrointestinal diseases) that could affect the results. The CS exclusion criteria were EDS of unknown origin or cataplexy, as established by the Epworth Sleepiness Scale and the Modified Cataplexy Emotional Trigger Questionnaire, respectively.
Autonomic dysfunction was evaluated using the Scales for Outcomes in Parkinson-Autonomic (SCOPA-AUT) and Parkinson's Disease Nonmotor Symptoms scales (PDNMS), which are both self-completed questionnaires. The University of Pennsylvania Smell Identification Test (UPSIT) was used for olfactory testing. Visual performance was first evaluated using Snellen charts and the Ishihara color test; color discrimination was then tested using the Farnsworth-Munsell 100 Hue Test (FM-100). The methodological details are reported elsewhere.5 RBD assessment was conducted using the RBD Screening Questionnaire (RBD-SQ), and emotional assessment was achieved with the short Beck Depression Inventory (BDI).
For statistical analyses, the Mann-Whitney U test was used for nonnormal endpoints (as assessed by a Shapiro-Wilk test) and ANOVA was used in cases of normality. All tests were two-tailed and the cutoff for statistical significance was set at p<0.05.
The primary outcomes were the following variables: total scores on the SCOPA-AUT, PDNMS, UPSIT, FM-100, RBD-SQ, and BDI. These primary variables were adjusted for multiplicity. All analyses were conducted using the Statistical Analysis System (SAS) statistical software (v9.2, SAS Institute, Cary, NC, USA). The study was approved by the National Ethical Committee in Luxembourg. All enrolled subjects provided written informed consent to participate prior to the study.
The demographic data were as follows: 10 males and 5 females for both the PN and the CS; age, 35.1±15.8 years for PN and 35.2±15.7 years for CS; and body mass index, 27.5±6.5 kg/m2 for PN and 24.4±4.3 kg/m2 for CS. Smoker status did not reach statistical significance (PN n=4, CS n=1). The clinical history was determined, and a clinical examination and polysomnography were performed to exclude any other concomitant cause for EDS in PN and CS (polysomnography was not performed in the CS). One of the PN suffered from sleep apnea syndrome, but was well controlled by continuous positive airway pressure treatment, as documented by polysomnography. None of the PN or CS was suffering from any other causes of EDS, such as insufficient sleep syndrome, shift work, or effects of substances or medications.
At study entry, the duration of narcolepsy was 13.6±5.6 years, as documented by the patient charts. Nine PN (four women) had cataplexy, while six PN (one woman) were without. Thirteen of the 15 PN were receiving specific treatment at study entry: modafinil (n=9), γ-hydroxybutyrate (n=1), and methylphenidate (n=1). The overall duration of treatment was 4.1±4.1 years.
All autonomic tests revealed significant differences between the PN and CS (Table 1, Fig. 1). There were significant differences between the two subject groups in SCOPA-AUT scores for all autonomic systems except for sexual disturbances. For the part of the questionnaire pertaining to sexual disturbances, the SCOPA-AUT scores for the male and female PN were 0.60±1.10 and 1.60±1.67, respectively, while those for their CS counterparts were both 0.00±0.00. The total PDNMS score was 10.53±4.78 for PN and 1.80±2.31 for CS. Autonomic dysfunction was significantly more common in PN than in CS.
Both the PN and CS exhibited hyposmia, as defined by the UPSIT test. The score did not differ significantly between PN (31.33±5.27) and CS (33.47±2.75, p=0.18). However, the distribution of the severity of hyposmia differed between the two groups, there being more markedly affected subjects among the PN than the CS (data not shown). Color discrimination was not different in the two groups, with FM-100 scores of 59.50±35.44 and 50.87±39.80 for PN and CS, respectively.
The findings of this study demonstrate that patients with narcolepsy also suffer from clinically relevant dysautonomia. The affected autonomic domains include pupillomotor function, the urinary, gastrointestinal, and cardiovascular systems, and thermoregulation. The SCOPA-AUT questionnaire, which was designed to assess nonmotor symptoms in IPD, provides the most concise evidence for these disturbances, while the pathological score on the PDNMS, which also assesses various sleep disturbances, is at least in part attributable to the intrinsic sleep dysfunction of NP. Neither of these instruments has yet been used in the assessment of PN. Both have been independently validated and are commonly recommended as screening tools for IPD patients (e.g., by the Movement Disorder Task Force on Rating Scales).6 The SCOPA-AUT questionnaire exhibits neither floor nor ceiling effects. Although not comparatively tested in this study, IPD patients typically achieve mean scores in both tests that are similar to those achieved by the PN in the present study.7 In both of these disease groups (i.e., patients with IPD and PN), urinary dysfunction scores are the highest, followed by those for gastrointestinal dysfunction in IPD patients and those for thermoregulation in PN.
Narcolepsy is commonly considered to be due to deficient hypocretin transmission, although reduced hypocretin levels in the CSF have routinely been evidenced only in PN with cataplexy. However, it is improbable that the pathophysiological cascade would be completely different in PN without cataplexy. CSF hypocretin levels were not assessed in the present cohort; we consider this to be a limitation of our study. We are also well aware that some dysautonomic complaints may be due to side effects of the medication. Despite these restrictions, we have (hypothetically) retained the concept that dysautonomia in narcolepsy may result from hypocretin deficiency. Below we briefly discuss both the pathophysiological and neuroanatomical data supporting this hypothesis. Early speculation regarding a putative connection between narcolepsy and dysautonomia were formulated without the knowledge of the hypocretin system. Nowadays there is more evidence of an association between narcolepsy and dysautonomia, although as recently pointed out, most studies are hampered by a lack of standardization or replication.8
The preganglionic neurons of the ciliary ganglion in the orbit are stimulated by hypocretin-containing nerve fibers. Via this pathway, emotional excitation and level of vigilance influence both the pupillary reaction to light and diurnal variations of the pupillary diameter.9 Reduced levels of hypocretin in narcolepsy could thus induce disturbances of the pupillomotor function and trigger increased sensitivity to light or, indirectly, disturbed vision. We therefore cannot exclude the possibility that the somnolence per se also impairs pupillomotor function in PN.
The findings of the present study show that PN often complain about constipation and premature satiation. Gastrointestinal disturbances have not been described in PN. The lateral hypothalamus, which contains hypocretin-producing neurons, is known to play a role in the acid secretion of the stomach, being particularly active during hunger periods. It stimulates acid secretion particularly during the cephalic phase, influenced by sensory stimuli. Furthermore, the vagal system is probably also controlled in part by the hypocretin system. Intraventricular application of hypocretin-1 in rats induces a dose-dependent increase in gastric acid secretion, an effect that is abolished by vagotomy or administration of atropine.10 Anatomically, the lateral hypothalamus is connected to the vagal system by the dorsal vagal complex and the dorsal motor nucleus (DMN). In rats, injection of hypocretin-1 in the DMN induces stomach contractions followed by stomach emptying.11
In the present cohort, PN frequently complained of orthostatic symptoms. It is known that PN exhibit attenuation of cardiovascular reflexes in comparison to healthy controls: during isometric muscular exercise, the increase in heart frequency in PN is reduced when compared to healthy controls. Respiratory sinus arrhythmia and heart rate are reduced during the Valsalva maneuver, and orthostatic counter-regulation is blunted after prolonged standing in PN.12 Finally, after arousals during REM and non-REM sleep, PN react with a reduced sympathetically driven heart rate increase in comparison to controls.13 In the rat, intraventricular application of hypocretin-1 increases blood pressure and serum levels of norepinephrine.14 Efferent hypocretinergic pathways target the rostral ventrolateral medulla (RVLM) and the intermediolateral spinal cord.15 Neurons of the RVLM control cardiovascular reflexes and sympathetic activity.16
Our NP also reported increased transpiration. Consistent with this, an increased distal-extremity diurnal body temperature has been observed in PN.17 In healthy subjects this can be observed at nighttime, with increased external temperatures or after prolonged standing. Artificially induced increases in body temperature are followed by impaired vigilance (somnolence) in PN.18 It is conceivable that increased body temperature may also contribute to daytime sleepiness in PN. The ventromedial medullary raphe nucleus (VMMR) contains presympathetic neurons for thermoregulation, which project to the lateral hypothalamus. Although there is no direct proof for the existence of hypocretin-containing neurons in the VMMR, it is well established that the lateral hypothalamus influences thermoregulation via regulation of the VMMR. This influence is most likely mediated via hypocretin-containing fibers.
The present data suggest that NP suffer from an increased frequency of urination during daytime or nighttime. In healthy subjects, urine production increases with acute sleep deprivation but slows down during sleep. It is thus conceivable that disturbed sleep at nighttime, as frequently observed in narcolepsy, induces increased urine production.
No significant differences between the two groups were found for libido and sexual function, although PN complained more often about sexual disturbances than CS. In a large cohort study, 21% of 347 NP indicated such dysfunction.19 These symptoms had only developed 10-20 years after disease onset. It is thus possible that the middle-aged PN of this cohort, who had suffered from narcolepsy for 14.2±6.2 years, may exhibit these symptoms at a later stage of their disease. Furthermore, a larger cohort might have revealed significant differences in this regard between the two groups.
Findings other than those regarding autonomic dysfunction in our NP need to be discussed. Depression has been reported frequently in PN.20 Reactive depression (to the disease) needs to be differentiated from a disease-inherent depression. Hypocretin modulates the circuitries involved in reward and stress management, and hypocretin-1 and -2 ionic channels influence 5-HT (serotonin)-producing neurons.21,22 Likewise, hyposmia is a well-recognized feature of narcolepsy.23 Hypocretin-containing nerve fibers diffusively innervate the olfactory network. Surprisingly, in our cohort hyposmia was present in both PN and CS. It is possible that the UPSIT test, which was primarily developed for American subjects, contains smell probes that are less familiar to European subjects (e.g., root beer and cheddar cheese) and thus not sufficiently discriminated by our cohort. The relatively high prevalence of hyposmia in our PN (60%) could be attributable to the small cohort; prevalence rates of 35-40% have been found in larger studies.23 Finally, smoker status may have been a substantial confounding factor, masking more subtle disease-inherent hyposmia in PN, although no significant differences were observed between the two groups. It is notable that none of our patients had a complete anosmia and none had noted the hyposmia previously. RBD is considered to be primarily due to hypocretin deficiency, possibly in the sublaterodorsal tegmental nucleus, one of the brainstem nuclei that regulates REM sleep. RBD prevalence among PN is increased in comparison to the general population.24 We found both a high and gender-neutral occurrence of RBD in our cohort using the RBD-SQ, which to the best of our knowledge has not yet been used in the assessment of PN. However, polysomnography did not confirm the diagnosis in this cohort. The high prevalence of RBD observed in our cohort of PN could also be due in part to their medical treatment. RBD occurs early in PN, and the mean age of our cohort was 35 years.24 In contrast, RBD is mostly a late-adult-life syndrome in patients with α-synucleinopathy, although it can precede the core Parkinsonian syndrome by decades.25
Finally, PN exhibit normal visual performance, and score similarly to CS in terms of color discrimination and contrast sensitivity. We believe that this finding is remarkable since it is the only one that distinguishes PN from patients with idiopathic RBD or patients with α-synucleinopathy in terms of "silent comorbidity," namely sensory deficits, depression, RBD, and in particular, dysautonomic signs. Thus, patients with idiopathic RBD-a possible precursor syndrome of IPD- and IPD patients, at an early stage of the disease, already suffer from EDS in addition to showing dysautonomic signs, hyposmia, RBD, and depression.4 Moreover, IPD patients exhibit significant disturbances of color and contrast discrimination, a finding that is absent in PN.4 Despite this notable discrepancy, we hypothesize that the complex syndrome identified in PN offers striking similarities with the multisystem involvement observed in IPD. The present data strongly suggest that beyond the classical tetrad of narcolepsy, patients with this disorder suffer from dysautonomic, depressive, and olfactory symptoms. It should be emphasized that such symptoms are often not declared by IPD patients to health professionals, although they strongly correlate with quality of life;26 the same may be true for PN.
Together the above-mentioned findings suggest that narcolepsy, although possibly triggered by an autoimmune process, nevertheless evolves into another progressive multisystem disease, a concept that is easily compatible with the diffuse connectivity of the hypocretin system, especially in the brainstem. Step-by-step progressive multisystem involvement in IPD has been suggested by Braak et al.27 However, there is a dearth of clinical data on the temporal evolution of dysautonomic and sensory deficits in narcolepsy, as well as postmortem studies to confirm or refute such a hypothesis.28
In addition to the symptom parallelism between IPD and narcolepsy, a direct pathochemical link has also been proposed based on pilot findings on decreased tissue levels of hypocretin and corresponding decreased numbers of hypocretin-containing neurons in IPD patients.29 However, the data remain controversial. Nevertheless, hypocretin deficiency has also been proposed in IPD to explain why some IPD patients exhibit EDS, even ahead of the motor manifestations of the disease, and why other patients react with EDS to the treatment with D3-dopamine agonists.30 Finally, it has been suggested that narcolepsy predisposes the patient to IPD. In a cohort of 1,152 PN, 3 later developed IPD, a rate that is 5 times higher than that in the general population. This raises the possibility that some factors intrinsic to narcolepsy or its treatment constitute risk factors for IPD.31
Some limitations of our study need to be mentioned. First, the sample was small and so the results must be replicated in larger cohorts. However, the results concerning the primary outcome variables remained robust after adjustment for multiple comparisons. The sample was too small to compare PN with and without cataplexy, and the CSF levels of hypocretin-1 were not determined. While alluding to similar deficits in IPD patients, a direct comparison with IPD patients was not performed, since an age effect would have confounded such an investigation. We did not perform objective autonomic tests to confirm the dysautonomia complaints assessed by the questionnaires. Finally, we were unable to evaluate patients who were not taking medication. Stimulants can modulate the autonomous nervous system, but acute withdrawal can also have a significant impact.32 Despite these limitations, we are confident that the findings of this exploratory study can be used to generate testable hypotheses, eventually anchoring narcolepsy within the spectrum of diseases with a multisystem impact.
In conclusion, The present data strongly suggest that beyond the classical tetrad of narcolepsy, patients with this disorder suffer from dysautonomic symptoms and confirm the comorbidities depression and RBD. The spectrum is comparable to the non-motor complex in IPD.
Figures and Tables
Fig. 1
Results of the PDNMS questionnaire in 15 patients with narcolepsy and 15 control subjects. apath: apathy, cardio: cardiovascular system, depre: depression, gastro: gastrointestinal system, hallu: hallucinations, misc: miscellaneous, PDNMS: Parkinson's Disease Non-motor Symptoms, sex: sexual system, sleep: sleep disturbances, urin: urinary system.
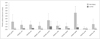
Table 1
Dysautonomia in narcolepsy

Data were presented as mean±SD values.
BDI: Beck Depression Inventory, f: female, FM-100: Farnsworth-Munsell 100 hue-test, m: male, n: number of subjects, PDNMS: Parkinson's Disease Nonmotor Symptoms, RBD-SQ: REM sleep behaviour disorder screening questionnaire, SCOPA-AUT: Scales for Outcomes in Parkinson-Autonomic, UPSIT: University of Pennsylvania Smell Identification Test.
References
1. Partinen M, Kornum BR, Plazzi G, Jennum P, Julkunen I, Vaarala O. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 2014; 13:600–613.

2. Nishino S, Kanbayashi T. Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system. Sleep Med Rev. 2005; 9:269–310.

3. Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996; 46:388–393.

4. Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006; 66:845–851.

5. Diederich NJ, Pieri V, Hipp G, Rufra O, Blyth S, Vaillant M. Discriminative power of different nonmotor signs in early Parkinson's disease. A case-control study. Mov Disord. 2010; 25:882–887.

6. Evatt ML, Chaudhuri KR, Chou KL, Cubo E, Hinson V, Kompoliti K, et al. Dysautonomia rating scales in Parkinson's disease: sialorrhea, dysphagia, and constipation--critique and recommendations by movement disorders task force on rating scales for Parkinson's disease. Mov Disord. 2009; 24:635–646.

7. Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson's disease: Results from an international pilot study. Mov Disord. 2007; 22:1901–1911.

8. Plazzi G, Moghadam KK, Maggi LS, Donadio V, Vetrugno R, Liguori R, et al. Autonomic disturbances in narcolepsy. Sleep Med Rev. 2011; 15:187–196.

9. Schreyer S, Büttner-Ennever JA, Tang X, Mustari MJ, Horn AK. Orexin-A inputs onto visuomotor cell groups in the monkey brainstem. Neuroscience. 2009; 164:629–640.

10. Takahashi N, Okumura T, Yamada H, Kohgo Y. Stimulation of gastric acid secretion by centrally administered orexin-A in conscious rats. Biochem Biophys Res Commun. 1999; 254:623–627.

11. Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005; 135:627–638.

12. Grimaldi D, Pierangeli G, Barletta G, Terlizzi R, Plazzi G, Cevoli S, et al. Spectral analysis of heart rate variability reveals an enhanced sympathetic activity in narcolepsy with cataplexy. Clin Neurophysiol. 2010; 121:1142–1147.

13. Sorensen GL, Knudsen S, Petersen ER, Kempfner J, Gammeltoft S, Sorensen HB, et al. Attenuated heart rate response is associated with hypocretin deficiency in patients with narcolepsy. Sleep. 2013; 36:91–98.

14. Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999; 831:248–253.

15. Dun NJ, Le Dun S, Chen CT, Hwang LL, Kwok EH, Chang JK. Orexins: a role in medullary sympathetic outflow. Regul Pept. 2000; 96:65–70.

16. McAllen RM, May CN, Shafton AD. Functional anatomy of sympathetic premotor cell groups in the medulla. Clin Exp Hypertens. 1995; 17:209–221.

17. Fronczek R, Overeem S, Lammers GJ, van Dijk JG, Van Someren EJ. Altered skin-temperature regulation in narcolepsy relates to sleep propensity. Sleep. 2006; 29:1444–1449.

18. Fronczek R, Raymann RJ, Romeijn N, Overeem S, Fischer M, van Dijk JG, et al. Manipulation of core body and skin temperature improves vigilance and maintenance of wakefulness in narcolepsy. Sleep. 2008; 31:233–240.

19. Roth B, Broughton RJ. Narcolepsy and Hypersomnia. Basel: Karger;1980.
20. Dodel R, Peter H, Spottke A, Noelker C, Althaus A, Siebert U, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007; 8:733–741.

21. Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res. 2007; 1154:163–172.

22. Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci. 2002; 22:8850–8859.

23. Bayard S, Plazzi G, Poli F, Serra L, Ferri R, Dauvilliers Y. Olfactory dysfunction in narcolepsy with cataplexy. Sleep Med. 2010; 11:876–881.

24. Nightingale S, Orgill JC, Ebrahim IO, de Lacy SF, Agrawal S, Williams AJ. The association between narcolepsy and REM behavior disorder (RBD). Sleep Med. 2005; 6:253–258.

25. Iranzo A, Santamaría J, Rye DB, Valldeoriola F, Martí MJ, Muñoz E, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology. 2005; 65:247–252.

26. Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, Mitra T, Frades-Payo B, Tluk S, et al. The nondeclaration of nonmotor symptoms of Parkinson's disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord. 2010; 25:704–709.

27. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003; 24:197–211.

28. Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009; 32:993–998.

29. Baumann C, Ferini-Strambi L, Waldvogel D, Werth E, Bassetti CL. Parkinsonism with excessive daytime sleepiness--a narcolepsy-like disorder? J Neurol. 2005; 252:139–145.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download