Abstract
Background
The survival and growth of melanocytes are controlled by the binding of stem cell factor to its cell surface receptor c-kit+ (CD117). We have observed that c-kit+ melanocytes existed in some lesions of vitiligo, while Melan A+ cells were absent.
Objective
To verify possible relation between c-kit+ expression and treatment response in non-segmental vitiligo lesions
Methods
Skin biopsies were done from the center of the 47 lesions from the 47 patients with non-segmental vitiligo. Expression of c-kit+ and Melan A, and amounts of melanin in the epidermis were assessed in each lesion, and treatment responses to excimer laser were evaluated.
There is as yet no agreement on the clinical evaluation system in vitiligo and the clinical course of nonsegmental vitiligo is variable123.
Usually, the onset of vitiligo is insidious and asymptomatic, and involves the formation of one or more macules initially1. Curiously, the exact appearance of early lesions is unclear, but it is probable that the 'early' macules gradually tend to lose pigment until it becomes completely white; it then enlarges centrifugally. It is generally believed that prompt treatment of 'early' lesions leads to better treatment response since melanocytes (MC) are still present in them12. However, this hypothesis has not been tested and indeed, the exact criteria of 'early' lesions are unclear. The survival of precursor MC or existence of some melanogenic functions of melanogenesis would well define the characteristics of 'early' thus responsive vitiliginous lesions rather than simple categorization by the duration of lesions. We have often seen the abundance of c-kit+ MC while Melan A+ MC were not present on skin biopsy of vitiligo. c-Kit+ seems to be expressed on the nonfunctional but survived MC.
The survival and/or growth of MC, and melanogenesis are controlled by the binding of stem cell factor (SCF) to its cell surface receptor c-kit+ (SCF receptor, CD117)456789. Interestingly, in previous studies, reduced expression of c-kit+ on MC is initiated by cytotoxic CD8+ T-lymphocytes7, which destabilize c-kit+ and lead to dysfunction of MC in hypopigmented mycosis fungoides56. Also, the presence of vitiligo in malignant melanoma has been reported, and c-kit+ is thought to be partly responsible for the dysfunction and loss of MC observed in vitiligo10. In addition, the expression of c-kit+ was reported to be essential for MC survival from radiation injury11.
To induce repigmentation in vitiligo, a 308 nm excimer laser have been successfully used for an average of 24 to 48 sessions21213. Ultraviolet B (UVB) irradiation augments the expression of membrane-bound SCF in epidermal keratinocytes, and the SCF activates neighboring MC via their kit receptors; thus for example, injection of kit-inhibitory antibody abolishes UVB-induced pigmentation14. In previous studies, decreased signaling via SCF/SCF receptor and diminished tyrosinase expression may cause complete loss of MC function at the lesional vitiligo skin15. In addition, even single UVB exposure stimulated the expression of kit in human MC via increasing level of microphthalmia-associated transcription factor (MITF)16.
Therefore, on these backgrounds, we hypothesized that (1) higher expression of c-kit+ is a good indicator of 'early' lesion of vitiligo and (2) the response of vitiligo to excimer laser may be related with the level of epidermal melanocytic expression of c-kit+ of vitiliginous lesions.
Patients who received a skin biopsy and excimer laser for non-segmental vitiligo at Asan Medical Center, Seoul, Korea, from March 2005 to December 2010 were enrolled in this study. Non-segmental vitiligo was diagnosed by physical examination and confirmed by histological examination from a punch biopsy of the center of the depigmented macules or patches. Excimer laser treatment was started at 5 to 7 days after the skin biopsy. To evaluate the response of excimer laser, patients who received regular treatment at least for 12 weeks (24 sessions) with excimer laser were included. Patients were excluded if they had a history of any previous (at least 3 months prior to the start of the study) treatment for vitiligo. The study was approved by the local ethical committee, and informed consent was obtained. This clinical study was approved by the Institutional Review Board of Asan Medical Center (IRB no. S2013-1483-0001).
In our study, we used excimer laser (PHAROS EX-308 excimer laser; Ra Medical Systems, Carlsbad, CA, USA) to treat patients with nonsegmental vitiligo. Since most patients had Fitzpatrick skin types III, or IV, the minimal erythema dose was not calculated. An initial dose of 100 mJ/cm2 was started in all patients and treatment was administered twice weekly for 12 weeks. The dose was increased by 20 mJ/cm2 weekly.
All patients were examined by the same dermatologist and photographs were taken at each visit to document the extent of repigmentation. All photographs were taken under strict and uniform conditions for constant settings. Response to treatment was assessed objectively by comparison of photographs taken before and at the 12 weeks (24 sessions of excimer laser) after the initiation of therapy. According to the extent of repigmentation relative to baseline status, lesions who responded to excimer laser therapy were classified into group A (good responder; ≥50% repigmentation, score 3 and 4), group B (moderate responder; 25%~50% repigmentation, score 2) and group C (poor responder; <25%, score 0 and 1). The clinical outcome score was defined to be 4 if repigmentation 75%~100%, 3 if repigmentation 50%~75%, 2 if repigmentation 25%~50%, 1 if repigmentation 0%~25%, and 0 when no repigmentation.
Skin biopsies were obtained from 47 lesional skin of the 47 patients and from nonlesional normal skin of the 5 patients. Hematoxylin and eosin (H&E), Fontana-Masson stainings were performed. Immunohistochemical staining was performed on 4-µm-thick, formalin-fixed, paraffin-embedded tissue sections using antibodies against Melan A (DAKO, Glostrup, Denmark), CD8 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and c-kit+ (CD117, Santa Cruz Biotechnology) in a Ventana autostaining system (Ventana Medical Systems Inc., Tucson, AZ, USA). Image analysis was performed using Image Pro Plus Version 4.5 (Media Cybernetics Co., Silver Spring, MD, USA). The amount of melanin was recorded as the percentage of pigmented area (PA) to measured epidermal area (EA) (PA/EA, ×100, %); Fontana-Masson staining value. Epidermal c-kit+ and CD8 expression was measured as the percentage of stained area (SA) to measured EA (SA/EA, ×100, %); c-kit+ staining value and CD8 staining value, respectively. The number of Melan A+ MC per 1 mm length of the rete ridge (1R) (MC/1R); Melan A staining value.
Statistical comparison of the results was performed by using Kruskal-Wallis test between group A, B, and C. Mann-Whitney U test was used to assess statistical significance of differences not only between normal skin and vitiligo lesions but between each group (group A, B, and C). Multiple logistic regression model was used to analyze the association between the degree of c-kit+ and CD8 staining and the effectiveness of excimer laser, adjusting for possible confounding factors. Statistical analyses were performed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). A significant probability less than 0.05 was considered statistically significant.
A total of 47 patients with nonsegmental vitiligo were diagnosed with skin biopsy and treated with excimer laser. The characteristics of the 47 patients are summarized in Table 1. Twenty-four patients (51.1%) were men and 23 (48.9%) were women; their ages ranged from 4~79 years with a mean age of 37.5 years. The duration of disease ranged from 1 to 120 months (mean 32.7 months). The location of lesion was extremities (38.3%, n=18), face and neck (31.9%, n=15) and trunk (29.8%, n=14).
Forty-seven patients were evaluated at the time after excimer laser treatment for 12 weeks. Repigmentation score of the lesions varied and recorded as follows. Forty-four patients (93.6%) were observed with repigmentation of vitiligo lesions and the other three patients presented no repigmentation. Classification of lesions was as follows; group A (31.9%, n=15), group B (34.0%, n=16) and group C (34.0%, n=16) (Table 2).
Duration of disease did not show significant difference between each group (p=0.490) (Table 2). Good repigmentation (≥50%, score 3 or 4) of lesions was more often seen in face/neck (40.0%) compared to those of the trunk (28.6%).
In vitiligo lesions, epidermal MC and melanin were markedly reduced and a mild perivascular lymphocytic infiltration was observed in H&E sections. Table 2 shows Fontana-Masson, Melan A, c-kit+, and CD8 staining values of vitiligo lesions.
Comparisons of the mean staining values in c-kit+, Fontana-Masson, Melan A, and CD8 between nonlesional normal skin and vitiligo lesions were shown in Table 3.
Fontana-Masson staining values in vitiliginous lesional epidermis ranged from 0 to 0.55 (mean 0.10). Melan A staining values in vitiliginous lesional epidermis ranged from 0 to 7.15 (mean 1.05). c-Kit+ staining values in vitiliginous lesional epidermis ranged from 0 to 5.97 (mean 1.39). And CD8 staining values in vitiliginous lesional epidermis were ranged from 0 to 3.78 (mean 0.35) (Table 3).
Compared to normal skin (Fontana-Masson 6.44±1.22, Melan A 11.90±1.17), vitiligo lesions (Fontana-Masson 0.10±0.15, Melan A 1.05±1.98) were seen with much less Fontana-Masson and Melan A+ cells (both p<0.001, Table 3). Fifteen of the 47 patients (31.9%) were good responders (group A) and they had higher c-kit+ staining value (mean 2.67±1.57). Sixteen of the 47 patients (34.0%) were poor responders (group C) and the lesions were measured with lower c-kit+ staining value (mean 0.61±0.54) (Table 2). There was apparent difference of c-kit+ staining value between good responders (group A) and the others (Fig. 1). Patients with stronger c-kit+ expression (measured values ≥1.5) had disease duration of less than 3 years (mean 29.5 months) and patients with weaker c-kit+ expression (measured values <1.5) had disease duration of more than 3 years (mean 37.5 months). These findings suggest 'good responder' vitiligo lesions showed significantly more expression of epidermal c-kit+ compared to 'poor responder' vitiligo lesions (p<0.001), and vitiligo patients with higher c-kit+ expression showed nonsignificant tendency of earlier stage of vitiligo (p=0.190). But the amount of melanin (Fontana-Masson), epidermal infiltration of CD8+ cells and the levels of Melan A which is used as markers of melanogenic activity of MC were not different between good responder (Fontana-Masson value 0.07±0.10, CD8 value 0.37±0.34, Melan A value 1.54±2.60) and poor responder (Fontana-Masson value 0.11±0.14, CD8 value 0.28±0.94, Melan A value 0.79±1.35) by image analyzer. The representative figure is shown in Fig. 2 which shows a representative pictures of the staining of Fontana-Masson, Melan A, and c-kit+ in normal skin, good responder (group A) and poor responder (group C) vitiligo lesions (Table 2). The multi-variable analysis for the association between the degree of c-kit+ and CD8 staining and good response after excimer laser treatment using multiple logistic regression was shown in Table 4. The degree of c-kit+ staining showed correlation with the clinical reponse, but the degree of CD8+ T cells infiltration did not.
Excimer laser and narrow band UVB treatment have remained the most effective treatment for vitiligo17. Although the mechanism is still unclear, UVB stimulates migration of MC from niches in the hair follicles, as well as their proliferation. UVB may also stimulate keratinocytes to secrete cytokines including SCF that act on MC, leading to melanogenesis. And it also upregulates expression of kit in MC1618. Several keratinocyte-derived factors including SCF are activators of MC and regulate skin pigmentation4. Decreased SCF expression by keratinocytes in vitiliginous skin is implicated in the apoptosis and loss of MC4. Kit (SCF receptor) is well known to play a regulatory role in normal human pigmentation, and dysfunction of kit results in several cutaneous depigmentary disorders, such as vitiligo14151618. Previous studies suggested that the reduced levels of c-kit+ and its downstream targets in MC are correlated with the dysfunction or loss of MC in vitiliginous epidermis151618. In our results using normal skin and vitiligo, there were c-kit+ + dermal cells which are composed of mostly dendritic cells and mast cells. c-Kit+ signaling is also known as important in erythropoiesis, lymphopoiesis, mast cell development and gametogenesis151618. In our study, epidermal c-kit+ expression was focused since in epidermis, MC or MC precursor cells have c-kit+ and the loss of c-kit+ is related to direct pathogenesis of vitiligo. In our study, 22 vitiligo lesions (46.8%) that we examined had levels (staining value of more than 1.0) of c-kit+ expressions similar to that observed in normal skin. In contrast, we found a reduced number of Melan A+ MC per 1R in vitiligo (mean 1.05±1.98) compared to normal skin (mean 11.90±1.17). c-kit+ expression remained to be high in vitiligo lesions where the number of MC is reduced in H&E staining and Melan A staining. Maintenance of c-kit+ level in the epidermis of lesional vitiligo was related with better treatment response to excimer laser. In the present study, the lesional epidermis of group A (good response at the 3 months of excimer laser) patients was positively stained with c-kit+. These fifteen patients (31.9%) had high level of c-kit+ + expression (mean 2.67±1.57) and correspondingly, the lesions achieved more than 50% of repigmentation. This suggests that the level of expression of c-kit+ and/or its downstream effectors in MC may be associated with the extent of pigmentation and response to treatment. Excimer laser treatment upregulates transcription and protein expression of SCF1618. Then, SCF binding to the c-kit+ induces MC proliferation and migration through phosphatidylinositol 3-kinase and Ras-MAPK pathway1920. In our opinion, the high level of expression of c-kit+ may have high potential of excimer laser induced upregulation of c-kit+. Further studies will be required to determine the specific mechanism of this phenomenon. The limitation of our study is that this is retrospective nature and the nonlesional skin biopsy could be obtained in only five patients because of possible scarring and possible Koebner phenomenon.
As early versus late vitiligo lesions are not easily distinguishable, our results may offer preliminary support for a possible role of c-kit+ levels as a marker of 'early' lesions of vitiligo which may have more benefit by early initiation of active treatment for better outcome.
A recent article insisted that if the early vitiligo lesions are done with excimer laser timely, the efficacy of the procedure can be increased21.
Decreased expression of c-kit+ protein and its downstream effectors, including MITF, a central transcription factor regulating the expression of tyrosinase mRNA in MC, may precede a loss of MC in vitiligo epidermis of late or stable stage222324.
In addition, there have been several reports demonstrating that there was a direct correlation among the severity of vitiligo, involvement of hair, and autoreactive CD8+ T cells in lesional vitiligo25. In our study, there were some degree of epidermal CD8+ cells but degree of CD8+ T cells infiltration did not show any correlation with the clinical response or duration of disease.
Our results showed that epidermal c-kit+ expression of vitiligo was related positively with clinical response to excimer laser while Melan A reactivity was already absent in the lesions. This suggests that active and prompt phototherapy is necessary for repigmentation when the SCF receptor function is still remained.
Figures and Tables
Fig. 1
There was apparent difference of c-kit+ staining value between good responders (group A) and the others by Mann-Whitney U test (p<0.05).

Fig. 2
Immunohistochemical staining for Fontana-Masson, Melan A, and c-kit+ (CD117) in sections of normal skin epidermis (A~C), good responder vitiligo lesions (D~F), and poor responder vitiligo lesion (G~I). Strong c-kit+ expression is present in normal epidermis (C) and moderate c-kit+ expression is present in good responder vitiligo lesional epidermis (F) compared to negative or almost negative c-kit+ expression in poor responder one (I). A~I: ×200.
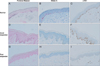
Table 1
Demographic characteristics of 47 vitiligo patients

| Characteristic | Value |
|---|---|
| Age (yr) | 37.5 (4~79) |
| Sex | |
| Male | 24 (51.1) |
| Female | 23 (48.9) |
| Disease duration (mo) | 32.7 (1~120) |
| Location of lesion | |
| Face/neck | 15 (31.9) |
| Trunk | 14 (29.8) |
| Extremities | 18 (38.3) |
Table 2
Clinical, histological and immunohistochemical characteristics

Table 3
Comparisons of the mean staining values in c-kit+, Fontana-Masson, Melan A, and CD8 between nonlesional normal skin and vitiligo lesions

Table 4
Multivariable analysis for the association between the degrees of c-kit+ and CD8 staining and good response after excimer laser treatment using multiple logistic regression

| Factors | Odds ratio (95% confidence interval) | p-value |
|---|---|---|
| Epidermal c-kit+ staining | 1.045 (1.015~1.075) | 0.003 |
| Epidermal CD8 staining | 0.996 (0.993~1.000) | 0.035 |
References
1. Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Edinburgh: Mosby Elsevier;2008.
2. Nicolaidou E, Antoniou C, Miniati A, Lagogianni E, Matekovits A, Stratigos A, et al. Childhood- and later-onset vitiligo have diverse epidemiologic and clinical characteristics. J Am Acad Dermatol. 2012; 66:954–958.

3. Agarwal S, Tamta M. Clinical predictors for the course of nonsegmental vitiligo. Clin Exp Dermatol. 2013; 38:669–670.

4. Singh ZN, Tretiakova MS, Shea CR, Petronic-Rosic VM. Decreased CD117 expression in hypopigmented mycosis fungoides correlates with hypomelanosis: lessons learned from vitiligo. Mod Pathol. 2006; 19:1255–1260.

5. El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, McCalmont TH. Hypopigmented mycosis fungoides: frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002; 26:450–457.
6. Steitz J, Wenzel J, Gaffal E, Tüting T. Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol. 2004; 83:797–803.

7. Mandelcorn-Monson RL, Shear NH, Yau E, Sambhara S, Barber BH, Spaner D, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol. 2003; 121:550–556.

8. Kasamatsu S, Hachiya A, Higuchi K, Ohuchi A, Kitahara T, Boissy RE. Production of the soluble form of KIT, s-KIT, abolishes stem cell factor-induced melanogenesis in human melanocytes. J Invest Dermatol. 2008; 128:1763–1772.

9. Richards KA, Fukai K, Oiso N, Paller AS. A novel KIT mutation results in piebaldism with progressive depigmentation. J Am Acad Dermatol. 2001; 44:288–292.

10. Kim YJ, Lee JB, Kim SJ, Lee SC, Won YH, Yun SJ. Amelanotic acral melanoma associated with KIT mutation and vitiligo. Ann Dermatol. 2015; 27:201–205.

11. Aoki H, Hara A, Motohashi T, Kunisada T. Protective effect of Kit signaling for melanocyte stem cells against radiation-induced genotoxic stress. J Invest Dermatol. 2011; 131:1906–1915.

12. Do JE, Shin JY, Kim DY, Hann SK, Oh SH. The effect of 308nm excimer laser on segmental vitiligo: a retrospective study of 80 patients with segmental vitiligo. Photodermatol Photoimmunol Photomed. 2011; 27:147–151.

13. Nicolaidou E, Antoniou C, Stratigos A, Katsambas AD. Narrowband ultraviolet B phototherapy and 308-nm excimer laser in the treatment of vitiligo: a review. J Am Acad Dermatol. 2009; 60:470–477.

14. Roskoski R Jr. Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005; 337:1–13.

15. Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, Kobayashi T, et al. Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol. 2004; 202:463–475.

16. Mizutani Y, Hayashi N, Kawashima M, Imokawa G. A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch Dermatol Res. 2010; 302:283–294.

17. Kawalek AZ, Spencer JM, Phelps RG. Combined excimer laser and topical tacrolimus for the treatment of vitiligo: a pilot study. Dermatol Surg. 2004; 30:130–135.

18. Katayama I, Ashida M, Maeda A, Eishi K, Murota H, Bae SJ. Open trial of topical tacalcitol [1 alpha 24(OH)2D3] and solar irradiation for vitiligo vulgaris: upregulation of c-Kit mRNA by cultured melanocytes. Eur J Dermatol. 2003; 13:372–376.
19. Jeon S, Kim NH, Kim JY, Lee AY. Stem cell factor induces ERM proteins phosphorylation through PI3K activation to mediate melanocyte proliferation and migration. Pigment Cell Melanoma Res. 2009; 22:77–85.

20. Lennartsson J, Blume-Jensen P, Hermanson M, Pontén E, Carlberg M, Rönnstrand L. Phosphorylation of Shc by Src family kinases is necessary for stem cell factor receptor/c-kit mediated activation of the Ras/MAP kinase pathway and c-fos induction. Oncogene. 1999; 18:5546–5553.

21. Kaliyadan F, Ashique KT. Optimizing the efficacy of targeted phototherapy by marking early vitiligo lesions after visualizing under a Wood's lamp. J Am Acad Dermatol. 2014; 71:e69.

22. Norris A, Todd C, Graham A, Quinn AG, Thody AJ. The expression of the c-kit receptor by epidermal melanocytes may be reduced in vitiligo. Br J Dermatol. 1996; 134:299–306.

23. Tachibana M, Takeda K, Nobukuni Y, Urabe K, Long JE, Meyers KA, et al. Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat Genet. 1996; 14:50–54.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download