Dear Editor:
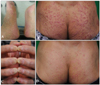
A 52-year-old male diagnosed with metastatic melanoma began treatment with 400 mg oral imatinib daily. The only other significant medical condition was a 20-year history of mild psoriasis, which was well-controlled with intermittent topical corticosteroid and calcipotriol ointment. The patient's pre-existing psoriasis did not require treatment for about two months before imatinib treatment was initiated. After two months of imatinib therapy, he presented with a painful, symmetric erythema on the palms and soles without any history of contact with irritants (Fig. 1A). Physical examination demonstrated the patient also developed not only palm and sole lesions but also erythematous, scaly papules to plaques of various sizes on the buttocks (Fig. 1B). He presented simultaneously with nail crumbling, pitting, onycholysis, beau lines, and subungual hyperkeratosis, which are typical features of psoriatic nail dystrophy (Fig. 1C). The patient had never experienced nail changes or any symptom of paronychia prior to this incident. For the painful palms and soles, hand-foot syndrome was diagnosed based on his clinical presentation. Since then, imatinib was discontinued and both palms and soles were treated with a short course of potent topical steroids. For the buttock lesions, a skin biopsy was performed.
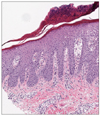
Two weeks later, the patients visit our clinics to check the result of biopsy, which suggested the exacerbation of underlying psoriasis (Fig. 2). Although palms and soles showed improvement, his buttock lesion kept aggravating when he visited the clinics. We then started topical corticosteroid and calcipotriol ointment. The withdrawal of imatinib treatment and application of topical corticosteroid and calcipotriol ointment gradually improved psoriatic skin lesions, and his skin condition was almost fully resolved after six weeks, but the nail changes have persisted (Fig. 1D). Imatinib administration was then re-initiated at the reduced dose of 200 mg daily; the skin lesions remain well-controlled.
Imatinib mesylate is a small-molecule compound that selectively inhibits multiple tyrosine kinases, including bcr-abl, c-kit, and platelet-derived growth factor receptor. Various cutaneous adverse reactions including nonspecific erythematous maculopapular rash, pityriasis rosea-like eruptions, and psoriasiform eruptions, have been reported1. In a review of published work, we found that there are several reports that imatinib may affect the pathogenesis of psoriasis. In most patients, psoriatic skin lesions occurred one to five months after 400 mg imatinib daily and improved upon withdrawal of imatinib and treatment with narrow-band ultraviolet B therapy. Topical corticosteroids with vitamin D analogues also facilitated improvement. After skin lesions resolved, dasatinib or nilotinib, a second-generation tyrosine kinase inhibitor, could be administered instead of imatinib Actually, second-generation tyrosine kinase was administered instead in two cases; subsequent psoriatic lesions were not reported23.
The mechanism behind imatinib-mediated psoriasis remains unknown. Woo et al.2 suggests a dose-dependent relationship, as his patient experienced exacerbation of underlying psoriasis after the imatinib dose was increased from 200 mg/day to 400 mg/day. The observed association between dose and psoriasis suggests that the immunological disturbance of psoriasis may be related to a pharmacological effect of imatinib. Previous studies showed that imatinib can block signaling pathways in regulatory T cells; further, the T-cell receptor may be blocked by imatinib due to inhibition of the phosphorylation of leucocyte-protein tyrosine kinase and reduced intracellular signaling in effector T cells45. Based on these results, Thachil5 proposed that "the balance between the regulatory and effector function of T cell" may determine the outcome of psoriasis; imatinib has been shown to interfere with this balance by blocking intracellular signaling.
The dose-dependent characteristics in this case suggest that pharmacological effects of imatinib play a pathological role in psoriasis. Further studies are needed to elucidate the precise mechanism by which imatinib induces this adverse cutaneous effect.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download