Dear Editor:
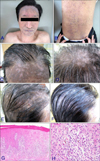
Lichenoid drug eruption (LDE) is an uncommon type of drug eruption that is rarely caused by antituberculosis drugs1. A 64-year old man visited our outpatient clinic in April 2012 due to pruritic skin eruption. Eight months previously, he was diagnosed with tuberculous meningitis, and had been initially treated for 2 months with isoniazid, moxifloxacin, ethambutol, and pyrazinamide, followed by alternative maintenance therapy with streptomycin, levofloxacin, and rifabutin were used for alternative maintenance therapy for the next 5 months. Ethambutol has been added to the drug regimen 4 weeks prior to the current visit. About 3 weeks prior to the present visit, pruritus and symmetrical violaceous polygonal flat papules developed on the whole body, especially on the forehead and scalp (Fig. 1A, B). Wickham's striae on their surface and oral mucosal involvement were not observed. During an early follow-up, scalp lesions rapidly developed into alopetic lesions (Fig. 1C~E). The patient had no previous history of skin diseases and pulmonary tuberculosis. The results of routine laboratory tests were within normal limits, except for 8.8% eosinophil in the blood. The histologic findings in the specimen from the back were consistent with LDE (Fig. 1G, H). Based on the clinical and histological results, a diagnosis of LDE was made. In the light of time-event relationship, ethambutol was assumed as the offending drug and so was discontinued. LDE was treated with oral antihistamines and topical 0.03% tacrolimus. Skin lesions improved markedly within 2 weeks, and gradually regressed during a 3-month follow-up period. Along with this improvement, scalp hairs began to regrow and a considerable portion of scalp became covered (Fig. 1F). There were no side effects of the topical tacrolimus during treatment periods.
In Korea, drug eruptions have occurred in 2.7 to 3.8% of outpatients2. LDE is a rare type (<1%) of drug eruption1, and clinical features are similar to lichen planus. However, some features have been described to be more characteristic of LDE; usually symmetrical lesions on the trunk, extremities; atypical morphology; no Wickham's striae; rare mucosal involvement; healing with residual hyperpigmentation; focal parakeratosis, hypogranulosis, and a superficial and deep perivascular infiltrate3; higher number of grouped necrotic keratinocytes; and infiltration of plasma cells and eosinophils4. Alopecia in association with LDE may be severe3 and has not been reported in the Korean dermatologic literature. Only one case report described scalp involvement without alopecia (Table 1). Treatments of LDE involve the elimination of the causative drug(s) and control of symptoms with antihistamines or topical steroids1. Oral lichen planus and oral lichenoid lesions have been successfully treated with tacrolimus5.
To our knowledge, this is the first reported case that shows prominent scalp alopetic lesion of LDE, and topical tacrolimus as an useful option to help improve these lesions. Although LDE initially involved the face and small parts of the scalp, we must keep in mind that these lesions can progress rapidly and result in severe scalp alopecia. When high potency topical steroids or long-term use of topical steroids are not allowed, topical tacrolimus is a wise option for the treatment of LDE.
Figures and Tables
Fig. 1
Large violaceous flat plaque on the forehead and small parts of scalp (A), and violaceous polygonal shaped flat papules on the back (B) were observed on initial visit. Two weeks later, scalp involvement progressed to alopetic lesions on frontal scalp (C), vertex (D) and parietal scalp (E). On microscopic examination, necrotic epidermal keratinocytes, lichenoid cellular infiltration of lymphocytes, histiocytes (G: H&E, ×40), and a few eosinophils in the papillary dermis (H: H&E, ×400) were observed. After 8 weeks' application of 0.03% topical tacrolimus and discontinuation of ethambtol, alopetic skin lesions improved (F) than before (E).
References
1. Na CH, Seo HD, Chung BS, Shin BS. A case of lichenoid drug eruption with whole body and oral mucosal involvement caused by antituberculosis drugs. Korean J Dermatol. 2008; 46:1145–1148.
2. Chang KY, Park HJ, Lim YS, Choi HY, Myung KB. Clinical study and skin tests of patients with drug eruptions. Korean J Dermatol. 1998; 36:997–1004.
3. Powell ML, Ehrlich A, Belsito DV. Lichenoid drug eruption to salsalate. J Am Acad Dermatol. 2001; 45:616–619.

4. Eom SC, Chae YS, Suh KS, Kim ST. The clinical features of lichenoid drug eruption and the histopathologic differentiation between lichenoid drug eruption and lichen planus. Korean J Dermatol. 1994; 32:1019–1025.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download