Dear Editor:
Terahertz (THz; 1 THz=1012 Hz≈33 cm-1) radiation, comprising electromagnetic waves propagating at frequencies in the THz range, lies between infrared and microwave radiation in the electromagnetic spectrum, and it shares some properties of each. Specifically, it travels in a straight line and is nonionizing. The THz spectral range typically covers frequencies between 0.1 and 10 THz, and THz radiation can interact with biological molecules, cells, and tissues1. However, until recently, this region of the electromagnetic spectrum has remained as a so-called THz-gap because of the lack of radiation sources. The recent advents of practical sources that cover the THz-gap have prompted its diverse applications including those in medical imaging2, telecommunication3, and security systems4. In the near future, people will be exposed to more and more THz radiation, and the exposure effects on skin will be a major concern because THz radiation is mostly absorbed by the skin due to its low penetrance of liquid water. A few studies have reported the effects of the millimeter waves on keratinocytes in vitro5-7; however, we do not know of any studies that have used in vivo models to examine the biological changes in the skin caused by THz radiation.
In this current study, the femtosecond (fs)-THz beamline at the Pohang Accelerator Laboratory was used as the THz light source8. The optical pulses were produced in a 3.2 W Ti:sapphire regenerative laser amplifier with a center wavelength of 800 nm and a pulse width of approximately 180 fs, which was delivered to a 10×10 mm <110> ZnTe crystal to generate fs-THz pulses via optical rectification. The fs-THz pulse has frequency up to 3 THz (wavelength, approximation 0.01 cm) and the pulse width of <200 fs with a repetition rate of 1 KHz. Before exposure, the THz pulse energy, measured with a Golay cell (Microtech Instruments Inc., Eugene, OR, USA) and a lock-in amplifier (SR830; Stanford Research Systems Inc., Sunnyvale, CA, USA), was approximately 0.26 nJ/pulse. To minimize the absorption by water vapor, a region of the THz beam path was enclosed and purged with dry air. A THz low-pass filter was used to remove the extra visible source during the process.
We exposed the back skin of 8-week-old male C57BL/6 mice to the fs-THz beam and examined the histological and molecular biological changes. Briefly, the hair on the back of the mice was clipped and they were positioned in the customized acryl restrainer manufactured for this experiment. After taking a precise position with the visible beam in targeting the spot, the back, the skin (size, 1×1 cm2) was exposed to the fs-THz beam for 1 hour. Accumulated energy in the targeted area for 1 hour was calculated as approximately 1.15 J/cm2. Skin biopsy samples were taken at 1 and 24 hours after exposure. Mice in the sham group were treated in the same manner except for the fs-THz beam exposure. Skin samples were stained with hematoxylin and eosin for histological assessment. We found that fs-THz irradiation did not induce any obvious histological changes in either 1 or 24 hours (Fig. 1). In addition, neither inflammatory cells nor damaged skin cells, such as sunburn cells, were observed. The collagen fibers in the dermis also seemed to be normal.
However, the quantitative real-time polymerase chain reaction analysis for the genes screened through microarray analysis reveals that some genes in the fs-THz-irradiated mice were either biologically activated or suppressed. For instance, the gene transcription of substance P (SP), a neuropeptide that is released from the C and Aδ fibers and is involved in local inflammation, including dermatoses, decreased significantly at 24 hours after exposure (Fig. 2A). On the contrary, the transcription of calcitonin gene-related peptide, another neuropeptide that is also associated with neurogenic inflammation in eczema, increased significantly in fs-THz-irradiated mice (Fig. 2B). We cannot provide a suitable explanation for this discrepancy because the biological effects of THz radiation are not yet fully understood. Moreover, the transcription of the transient receptor potential vanilloid (TRPV) 1 and 4, which are kinds of ion channels, seemed to decrease at 24 hours after exposure, but the changes were not significant due to the small sample size (Fig. 2C, D). Further, we performed the same experiment on BALB/c nude mice, in which they were exposed to the fs-THz beam for 1 hour, and biopsy samples were taken at 12 and 36 hours after exposure. We observed that the transcription of the TRPV1 gene decreased significantly at 36 hours after exposure (Fig. 2E). Interestingly, TRPV1 has been reported to be able to release SP9; therefore, it could be hypothesized that fs-THz pulses exert their biological effects primarily through the modulation of ion channels. However, further investigation is necessary to confirm this.
In conclusion, our data show that fs-THz pulses have biological effects at the molecular level, even though the mean energy of the radiation was quite low to induce any noticeable histological changes. This study has some limitations; THz radiation at different frequencies, power, and pulse durations were not studied, and it is difficult to presume the clinical meaning of this study. Moreover, we did not test higher energy of THz radiation because increasing the exposure time was difficult due to the limit of anesthesia. In spite of these limitations, this is the first study to demonstrate the molecular biological effects of THz radiation on the skin using an animal model to the best of our knowledge, and we hope that our results promote further investigations in the future.
Figures and Tables
Fig. 1
Histological observations on the back skin of C57BL/6 mice exposed to the femtosecond-Terahertz (fs-THz) beam for 1 hour (H&E; ×200 and ×400). Skin samples were biopsied at 1 and 24 hours after exposure.
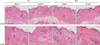
Fig. 2
Gene expression in the femtosecond-Terahertz (fs-THz)-irradiated mouse skin. The back skin of C57BL/6 mice (sham, n=4; fs-THz, n=5; A~D) and BALB/c nude mice (sham, n=3; fs-THz, n=4; E~F) were exposed to the fs-THz beam for 1 hour. Skin samples were biopsied either at 1 and 24 hours after exposure of C57BL/6 mice or at 12 and 36 hours after exposure of BALB/C nude mice. Real-time quantitative polymerase chain reaction was used for gene expression survey: (A) substance P, (B) calcitonin gene-related peptide, (C, E) transient receptor potential vanilloid1 (TRPV1), (D, F) TRPV4. Results were expressed as mean±standard error of the mean. *p<0.05, compared to the nonirradiated sham group.

ACKNOWLEDGMENT
This study was supported by the National Research Foundation of Korea; grants were funded by the Korean Government (MSIP, 2009-0083512), and the Agency of Defense Development (2009-0093427).
References
1. Smye SW, Chamberlain JM, Fitzgerald AJ, Berry E. The interaction between Terahertz radiation and biological tissue. Phys Med Biol. 2001; 46:R101–R112.

2. Joseph CS, Yaroslavsky AN, Neel VA, Goyette TM, Giles RH. Continuous wave terahertz transmission imaging of nonmelanoma skin cancers. Lasers Surg Med. 2011; 43:457–462.

3. Möller L, Federici J, Sinyukov A, Xie C, Lim HC, Giles RC. Data encoding on terahertz signals for communication and sensing. Opt Lett. 2008; 33:393–395.

4. Grossman E, Dietlein C, Ala-Laurinaho J, Leivo M, Gronberg L, Gronholm M, et al. Passive terahertz camera for standoff security screening. Appl Opt. 2010; 49:E106–E120.

5. Szabo I, Manning MR, Radzievsky AA, Wetzel MA, Rogers TJ, Ziskin MC. Low power millimeter wave irradiation exerts no harmful effect on human keratinocytes in vitro. Bioelectromagnetics. 2003; 24:165–173.

6. Szabo I, Rojavin MA, Rogers TJ, Ziskin MC. Reactions of keratinocytes to in vitro millimeter wave exposure. Bioelectromagnetics. 2001; 22:358–364.

7. Chen Q, Zeng QL, Lu DQ, Chiang H. Millimeter wave exposure reverses TPA suppression of gap junction intercellular communication in HaCaT human keratinocytes. Bioelectromagnetics. 2004; 25:1–4.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download