Abstract
Hepatocellular carcinoma (HCC) is a highly malignant tumor that has limited treatment options in its advanced state. The efficacy of current anti-cancer chemotherapy in advanced HCC has not been satisfactory, and the management of HCC remains a major challenge for physicians. However, recent advancement in our understanding of the molecular mechanisms underlying carcinogenesis has provided novel targets in key signaling pathways for tumor development. Recently, Sorafenib (Nexavar), a multi-kinase inhibitor targeting membrane receptors involved in angiogenic and mitogenic intra-cellular signaling including Raf, vascular endothelial growth factor receptor (VEGFR), and platelet-derived growth factor receptors (PDGFR), has been approved worldwide as a new target agent for HCC, and subsequent clinical trials of newly developed molecular target agents such as Brivanib and Everolimus are being conducted globally. In the near future, continued research will lead to the identification of additional molecular targets in HCC and lead to treatments with enhanced efficacy and improved tolerability.
Figures and Tables
Fig. 1
The etiologic factors and pathogenesis of hepatocellular carcinoma. HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; AFB1, aflatoxin B1; p53, tumor suppressor gene p53.
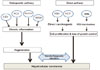
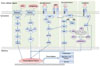
Fig. 2
Signaling pathways in hepatocarcinogenesis. Wnt/Hedgehog pathway, growth factor pathway, survival factor pathway, Akt/mTOR pathway, and Jak/stat pathway are involved in the hepatocarcinogenesis, and can be targeted for HCC therapy. DSH, Dishevelled; GBP,GSK3B binding protein; GSK3B, glycogen synthase kinase 3-beta; APC, adenomatous polyposis coli; PTCH1, PATCHED1 membrane protein; SMOH, Smoothened; SU, suppressor-of-fused; COS-2, Drosophila Costal2; FU, Fused; PI3K, phosphoinositide 3-kinase; GLI, glioblastoma; RTK, receptor tyrosine kinase; SOS, son of sevenless homolog; Grb2, growth factor receptor-bound protein 2; MAP2K, mitogen-activated protein kinase kinase; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3 kinase; PKC, protein kinase C; AKT, v-akt murine thymoma viral oncogene homolog; PTEN, phosphatase and tensin homolog; Bcl XL, B-cell lymphoma extra large.
References
1. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007. 132:2557–2576.

2. Poon D, Anderson BO, Chen LT, et al. Management of hepatocellular carcinoma in Asia: Consensus statement from the Asian Oncology Summit. 2009. Lancet Oncol. 2009. 10:1111–1118.

3. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010. 30:61–74.

4. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008. 359:378–390.

5. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010. 15:Suppl 4. 14–22.

6. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006. 6:674–687.

7. Wysocki PJ. Targeted therapy of hepatocellular cancer. Expert Opin Investig Drugs. 2010. 19:265–274.

8. Kudo M. Signaling pathway and molecular-targeted therapy for hepatocellular carcinoma. Dig dis. 2011. 29:289–302.

9. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. New Engl J Med. 2008. 358:1160–1174.

10. Buckley AF, Burgart LJ, Sahai V, Kakar S. Epidermal growth factor receptor expression and gene copy number in conventional hepatocellular carcinoma. Am J Clin Pathol. 2008. 129:245–251.

11. Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005. 23:6657–6663.

12. Thomas MB, Chadha R, Glover K, Wang X, Morris J, Brown T, et al. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007. 110:1059–1067.

13. Philip PA, Mahoney MR, Holen KD, Northfelt DW, Pitot HC, Picus J, et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer. 2012. 118:2424–2430.

14. Thomas MB, Morris JS, Chadha R, Iwasaki M, Kaur H, Lin E, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009. 27:843–850.

15. Burris HA 3rd, Hurwitz HI, Dees EC, Dowlati A, Blackwell KL, O'Neil B, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005. 23:5305–5313.

16. Ramanathan RK, Belani CP, Singh DA, Tanaka M, Lenz HJ, Yen Y, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009. 64:777–783.

17. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006. 354:567–578.

18. Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007. 357:2040–2048.
19. Zhu AX, Stuart K, Blaszkowsky LS, Muzikansky A, Reitberg DP, Clark JW, et al. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007. 110:581–589.
20. Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004. 4:505–518.

21. Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006. 25:3787–3800.

22. Scharf JG, Braulke T. The role of the IGF axis in hepatocarcinogenesis. Horm Metab Res. 2003. 35:685–693.

23. Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004. 4:335–348.

24. Wang L, Wang WL, Zhang Y, Guo SP, Zhang J, Li QL. Epigenetic and genetic alterations of PTEN in hepatocellular carcinoma. Hepatol Res. 2007. 37:389–396.

25. Schmitz KJ, Wohlschlaeger J, Lang H, Sotiropoulos GC, Malago M, Steveling K, et al. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol. 2008. 48:83–90.

26. Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006. 6:729–734.

27. Rizell M, Andersson M, Cahlin C, Hafstrom L, Olausson M, Lindner P. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol. 2008. 13:66–70.

28. Huynh H, Chow KH, Soo KC, Toh HC, Choo SP, Foo KF, et al. RAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med. 2009. 13:1371–1380.

29. Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007. 72:Suppl 1. 30–44.

30. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007. 26:3279–3290.

31. Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006. 130:1117–1128.

32. Jenke HS, Deml E, Oesterle D. C-raf expression in early rat liver tumorigenesis after promotion with polychlorinated biphenyls or phenobarbital. Xenobiotica. 1994. 24:569–580.
33. Beer DG, Neveu MJ, Paul DL, Rapp UR, Pitot HC. Expression of the c-raf protooncogene, gamma-glutamyltranspeptidase, and gap junction protein in rat liver neoplasms. Cancer Res. 1988. 48:1610–1617.
34. Hwang YH, Choi JY, Kim S, Chung ES, Kim T, Koh SS, et al. Over-expression of c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular carcinoma. Hepatol Res. 2004. 29:113–121.

35. Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008. 7:3129–3140.

36. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009. 10:25–34.

38. Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008. 7:504–516.

39. Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, et al. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002. 20:1775–1785.

40. Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005. 4:220–226.

41. Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer. 2009. 100:1385–1392.

42. Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008. 26:2992–2998.

43. Finn RS, Kang YK, Mulcahy M, Polite BN, Lim HY, Walters I, et al. Phase II, Open-label Study of Brivanib as Second-line Therapy in Patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res. 2012. 18:2090–2098.

44. Park JW, Finn RS, Kim JS, Karwal M, Li RK, Ismail F, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011. 17:1973–1983.

45. Kanai F, Yoshida H, Tateishi R, Sato S, Kawabe T, Obi S, et al. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2011. 67:315–324.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download