Abstract
Figures and Tables
Figure 1

Figure 2
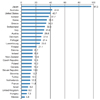
Figure 3

Figure 4

Figure 5

Table 1
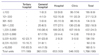
Values are presented as number (%).
Tests covered by National Health Insurance were counted.
From Choi YJ, et al. Development of fee schedule for high-tech medical imaging devices. Seoul: Health Insurance Review Agency; 2012 [6].
Table 2
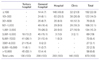
Values are presented as number (%).
Tests covered by National Health Insurance were counted.
From Choi YJ, et al. Development of fee schedule for high-tech medical imaging devices. Seoul: Health Insurance Review Agency; 2012 [6].
Table 3

Values are presented as number (%).
From Han KH, et al. Korean J Hosp Manag 2007;12:31-50, according to the Creative Commons license [8].
a)Angio, computed radiography . digital radiography, mammogram, gamma-camera, computed tomography, magnetic resonance imaging, extracorporeal shock wave lithotripsy, positron emission tomography, linear accelerator, and gamma-knife.
Table 4
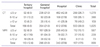
Values are presented as number (%).
Units of which production year are unknown were excluded.
From Han KH, et al. Korean J Hosp Manag 2007;12:31-50, according to the Creative Commons license [8].




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download