Abstract
Due to the development of dedicated receiver coils for 3 tesla (T) magnetic resonance (MR) imaging and increased gradient performance, 3T MR imaging of the abdomen is rapidly becoming a part of routine clinical practice. The most important advantage of 3T MR imaging is a higher signal-to-noise ratio and contrast-to-noise ratio compared with 1.5T systems, which can be used to improve spatial resolution and shorten image acquisition time. In the abdomen, the improved image quality of non-enhanced and enhanced solid organ imaging, MR angiography, MR cholangiopancreatography, and MR spectroscopy can be obtained at 3T due to the increased signal-to-noise ratio and contrast-to-noise ratio. However, 3T abdominal MR imaging also presents several technical challenges, such as increased energy deposition within the patient's body, standing wave artifacts, and increased susceptibility artifacts. Therefore, abdominal MR imaging at 3T requires adjustments in the sequence parameters of pulse sequences designed for 1.5T to optimize image quality. At present, 3T abdominal MR imaging is feasible with high image quality in an acceptable scan time, but 3T imaging is not significantly superior to 1.5T imaging in terms of cost-effectiveness. Future improvements in coil technology and new sequences suitable for 3T may enable wider clinical use of 3T for abdominal MR imaging.
Figures and Tables
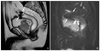
Figure 1
Comparison of T2-weighted fast spin echo images obtained with 1.5 tesla (T) (A) and 3T (B) systems in the same patient. The image obtained at 3T shows an increased signal-to-noise ratio with increased conspicuity of a small hepatic cyst (arrow). Parameters at 1.5T were 5,500/88 (repetition time msec/echo time msec), 6-mm section thickness, 320×224 matrix, and 36-cm field of view. Parameters at 3T were 2,500/99, 6-mm section thickness, 512×255 matrix, and 36-cm field of view.
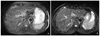
Figure 2
High-resolution isotropic 3D T2-weighted images of the female pelvis at 3 tesla (T). (A) 3D turbo spin echo T2-weighted image in a 58-year-old woman with cervical cancer shows slightly hyperintense endocervical mass (arrow). Sequence parameters were sampling perfection with application of optimized contrasts using different flip angle evolution (SPACE), 2,000/127, 1-mm section thickness, echo train of 89, 256×256 matrix, and 18-cm field of view. (B) Sagittal reconstructed image from 3D data set again shows hyperintense endocervical mass (arrow) and two nabothian cysts (arrowheads). B, bladder; R, rectum.
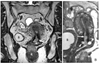
Figure 3
Increased conspicuity of hepatic metastasis at 3 tesla (T) magnetic resonance imaging using liver-specific contrast agent in a pa-tient with rectal cancer. (A) Contrast-enhanced computed tomography shows a subtle low density lesion (arrow) at the periphery of the right hepatic lobe. (B) Axial dynamic contrast-enhanced 3D gradient echo T1-weighted image with fat saturation after injection of gadoxetic acid shows a small low signal intensity lesion with peripheral rim enhancement on hepatic arterial phase. (C) On hepatobiliary phase obtained 20 minutes after contrast injection, the mass shows distinct low signal intensity (arrow) compared with background liver enhancement. Another unexpected small metastatic lesion (arrowhead) is also easily detected the segment 7 of the liver.

Figure 4
Maximum-intensity projection image from 3 tesla abdominal magnetic resonance angiographic data obtained from a 52-year-old woman with kidney transplantation demonstrates excellent contrast-to-noise ratio and detailed vascular anatomy.
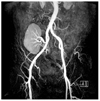
Figure 5
Coronal maximum-intensity projection image from 3D magnetic resonance cholangiopancreatography at 3 tesla shows increased fluid conspicuity due to a high signal-to-noise ratio in a 42-year-old man with pancreas head adenocarcinoma. Abrupt cut-off of the distal common bile duct and pancreatic duct at the head portion (arrow) is well demonstrated.
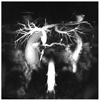
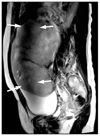
Figure 6
Increased susceptibility artifacts at 3 tesla in a 64-year-old man with proximal rectal cancer. Pelvic magnetic resonance images were obtained after endoscopic biopsy and metallic clipping. Sagittal T2-weighted image (A) and diffusion-weighted image (B) (b=500 sec/mm2) show prominent susceptibility artifacts around the metallic clip (arrows). It is difficult to know the exact extent of the rectal cancer due to the severe artifacts.
References
1. Merkle EM, Dale BM, Paulson EK. Abdominal MR imaging at 3T. Magn Reson Imaging Clin N Am. 2006. 14:17–26.

2. Barth MM, Smith MP, Pedrosa I, Lenkinski RE, Rofsky NM. Body MR imaging at 3.0 T: understanding the opportunities and challenges. Radiographics. 2007. 27:1445–1462.

3. Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008. 28:1983–1998.

4. Merkle EM, Dale BM. Abdominal MRI at 3.0 T: the basics revisited. AJR Am J Roentgenol. 2006. 186:1524–1532.

5. Katz-Brull R, Rofsky NM, Lenkinski RE. Breathhold abdominal and thoracic proton MR spectroscopy at 3T. Magn Reson Med. 2003. 50:461–467.

6. Soher BJ, Dale BM, Merkle EM. A review of MR physics: 3T versus 1.5T. Magn Reson Imaging Clin N Am. 2007. 15:277–290.

7. Akisik FM, Sandrasegaran K, Aisen AM, Lin C, Lall C. Abdominal MR imaging at 3.0 T. Radiographics. 2007. 27:1433–1444.

8. Erturk SM, Alberich-Bayarri A, Herrmann KA, Marti-Bonmati L, Ros PR. Use of 3.0-T MR imaging for evaluation of the abdomen. Radiographics. 2009. 29:1547–1563.

9. Hussain SM, Wielopolski PA, Martin DR. Abdominal magnetic resonance imaging at 3.0 T: problem or a promise for the future? Top Magn Reson Imaging. 2005. 16:325–335.
10. Martin DR, Friel HT, Danrad R, De Becker J, Hussain SM. Approach to abdominal imaging at 1.5 Tesla and optimization at 3 Tesla. Magn Reson Imaging Clin N Am. 2005. 13:241–254.

11. Krautmacher C, Willinek WA, Tschampa HJ, Born M, Träber F, Gieseke J, Textor HJ, Schild HH, Kuhl CK. Brain tumors: full- and half-dose contrast-enhanced MR imaging at 3.0 T compared with 1.5 T. Initial Experience. Radiology. 2005. 237:1014–1019.

12. Tsurusaki M, Semelka RC, Zapparoli M, Elias J Jr, Altun E, Pamuklar E, Sugimura K. Quantitative and qualitative comparison of 3.0T and 1.5T MR imaging of the liver in patients with diffuse parenchymal liver disease. Eur J Radiol. 2009. 72:314–320.

13. Ramalho M, Altun E, Herédia V, Zapparoli M, Semelka R. Liver MR imaging: 1.5T versus 3T. Magn Reson Imaging Clin N Am. 2007. 15:321–347.

14. Campeau NG, Huston J 3rd, Bernstein MA, Lin C, Gibbs GF. Magnetic resonance angiography at 3.0 Tesla: initial clinical experience. Top Magn Reson Imaging. 2001. 12:183–204.

15. Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005. 40:715–724.

16. Michaely HJ, Nael K, Schoenberg SO, Finn JP, Laub G, Reiser MF, Ruehm SG. The feasibility of spatial high-resolution magnetic resonance angiography (MRA) of the renal arteries at 3.0 T. Rofo. 2005. 177:800–804.

17. Prince MR, Zhang HL, Prowda JC, Grossman ME, Silvers DN. Nephrogenic systemic fibrosis and its impact on abdominal imaging. Radiographics. 2009. 29:1565–1574.

18. Perez-Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment: report of 33 cases. Radiology. 2009. 250:371–377.

19. Schindera ST, Miller CM, Ho LM, DeLong DM, Merkle EM. Magnetic resonance (MR) cholangiography: quantitative and qualitative comparison of 3.0 Tesla with 1.5 Tesla. Invest Radiol. 2007. 42:399–405.
20. Isoda H, Kataoka M, Maetani Y, Kido A, Umeoka S, Tamai K, Koyama T, Nakamoto Y, Miki Y, Saga T, Togashi K. MRCP imaging at 3.0 T vs. 1.5 T: preliminary experience in healthy volunteers. J Magn Reson Imaging. 2007. 25:1000–1006.

21. Choi JY, Kim MJ, Chung YE, Kim JY, Jones AC, de Becker J, van Cauteren M. Abdominal applications of 3.0-T MR imaging: comparative review versus a 1.5-T system. Radiographics. 2008. 28:e30.

22. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007. 188:1622–1635.

23. Naganawa S, Kawai H, Fukatsu H, Sakurai Y, Aoki I, Miura S, Mimura T, Kanazawa H, Ishigaki T. Diffusion-weighted imaging of the liver: technical challenges and prospects for the future. Magn Reson Med Sci. 2005. 4:175–186.

24. Notohamiprodjo M, Dietrich O, Horger W, Horng A, Helck AD, Herrmann KA, Reiser MF, Glaser C. Diffusion tensor imaging (DTI) of the kidney at 3 tesla-feasibility, protocol evaluation and comparison to 1.5 Tesla. Invest Radiol. 2010. 45:245–254.

25. Jacobs MA, Ouwerkerk R, Petrowski K, Macura KJ. Diffusion-weighted imaging with apparent diffusion coefficient mapping and spectroscopy in prostate cancer. Top Magn Reson Imaging. 2008. 19:261–272.

26. Wang J, Yu T, Bai R, Sun H, Zhao X, Li Y. The value of the apparent diffusion coefficient in differentiating stage IA endometrial carcinoma from normal endometrium and benign diseases of the endometrium: initial study at 3-T magnetic resonance scanner. J Comput Assist Tomogr. 2010. 34:332–337.

27. Namimoto T, Awai K, Nakaura T, Yanaga Y, Hirai T, Yamashita Y. Role of diffusion-weighted imaging in the diagnosis of gynecological diseases. Eur Radiol. 2009. 19:745–760.

28. Lin G, Ng KK, Chang CJ, Wang JJ, Ho KC, Yen TC, Wu TI, Wang CC, Chen YR, Huang YT, Ng SH, Jung SM, Chang TC, Lai CH. Myometrial invasion in endometrial cancer. Diagnostic accuracy of diffusion-weighted 3.0-T MR imaging: initial experience. Radiology. 2009. 250:784–792.

29. Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, Choi BI. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009. 253:116–125.

30. Goshima S, Kanematsu M, Kondo H, Yokoyama R, Kajita K, Tsuge Y, Watanabe H, Shiratori Y, Onozuka M, Moriyama N. Diffusion-weighted imaging of the liver: optimizing b value for the detection and characterization of benign and malignant hepatic lesions. J Magn Reson Imaging. 2008. 28:691–697.

31. Zech CJ, Herrmann KA, Dietrich O, Horger W, Reiser MF, Schoenberg SO. Black-blood diffusion-weighted EPI acquisition of the liver with parallel imaging: comparison with a standard T2-weighted sequence for detection of focal liver lesions. Invest Radiol. 2008. 43:261–266.

32. Shellock FG, Crues JV. MR procedures: biologic effects, safety, and patient care. Radiology. 2004. 232:635–652.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download