Abstract
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by selective autoimmune-mediated destruction of pancreatic islet beta-cells leading gradually to absolute insulin deficiency. T1D is under polygenic control. The HLA complex attributes 50% of the genetic risk for T1D while as many as 20 genes influence susceptibility to T1D. The autoimmune beta-cell destruction could be triggered by environmental factors. While the exact trigger of anti-islet autoimmunity remains elusive, it can lead to an imbalance between regulatory T cells and autoimmune effector T cells. During the initiation of insulitis, emerging evidences suggest that the infiltrating macrophages via toll-like receptor 2 (TLR2) activation lead to induction and amplification of insulitis. Following the priming of diabetogenic T-cells, autoreactive T effector cells destroy the beta cells by direct contact- dependent cytolysis or by soluble mediators secreted from macrophages or CD4 T effector cells. The hyperglycemia occurs late in its course after 80% of the beta cells have been destroyed. Although no current cure exists, refinement of genetic studies and islet autoantibodies has improved the ability to predict the risk of T1D and aid the establishment of rationally designed preventive therapies. Other strategies involve beta-cell replacement by islet transplantation. Extensive and long-term research on the efficacy of islet transplantation and preservation of beta-cell function is keenly needed.
References
2. Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003; 198:1527–1537.
3. Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007; 27:321–333.
4. Suarez-Pinzon WL, Power RF, Rabinovitch A. Fas ligand-mediated mechanisms are involved in autoimmune destruction of islet beta cells in non-obese diabetic mice. Diabetologia. 2000; 43:1149–1156.

5. Kim S, Kim K-A, Hwang D-Y, Lee TH, Kayagaki N, Yagita H, Lee M-S. Inhibition of autoimmune diabetes by Fas ligand: the paradox is solved. Journal of Immunology. 2000; 164:2931–2936.

6. Yoon JW, Jun HS, Santamaria P. Cellular and molecular mechanisms for the initiation and progression of beta cell destruction resulting from the collaboration between macrophages and T cells. Autoimmunity. 1998; 27:109–122.
7. Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev. 2008; 29:334–350.
8. http://www.immunetolerance.org
9. Callewaert HI, Gysemans CA, Ladriere L, D'Hertog W, Hagenbrock J, Overbergh L, Eizirik DL, Mathieu C. Deletion of STAT-1 pancreatic islets protects against streptozotocin-induced diabetes and early graft failure but not against late rejection. Diabetes. 2007; 56:2169–2173.

10. Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, Klein T, Serup P, Eizirik DL, Melloul D. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA. 2006; 103:5072–5077.
11. Suk K, Kim S, Kim Y-H, Kim K-A, Chang I, Yagita H, Shong M, Lee M-S. IFNg/TNFa Synergism as the Final Effector in Autoimmune Diabetes: A key role for STAT1/IRF-1 in pancreatic b-cell death. Journal of Immunology. 2001; 166:4481–4489.
12. Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS. NF-kappa B prevents beta cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci USA. 2007; 104:1913–1918.
13. Standards of medical care in diabetes-2009. Diabetes Care. 2009; 32(Suppl 1):13–61.
14. Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008; 359:2767–2777.

15. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of longterm complications in insulindependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.
16. http://www.diabetestrialnet.org
17. Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1 (Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. N Engl J Med. 2005; 54:1763–1769.
18. Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Cases R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008; 359:1909–1920.

19. Bollyky J, Sanda S, Greenbaum CJ. Type 1 diabetes mellitus: primary, secondary, and tertiary prevention. Mt Sinai J Med. 2008; 75:385–397.

20. Skyler JS. Update on worldwide efforts to prevent type 1 diabetes. Ann N Y Acad Sci. 2008; 1150:190–196.

21. Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002; 346:1685–1691.
22. Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial-Type 1. Diabetes Care. 2005; 28:1068–1076.
23. Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004; 363:925–931.

24. Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N Engl J Med. 2000; 343:230–238.

25. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio E, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006; 355:1318–1330.

27. D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006; 24:1392–1401.
28. Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, Mendez AJ, Selvaggi G, Ricordi C, Alejandro R. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008; 86:1658–1665.

29. Froud T, Faradji RN, Pileggi A, Messinger S, Baidal DA, Ponte GM, Cure PE, Monroy K, Mendez A, Selvaggi G, Ricordi C, Alejandro R. The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy, and metabolic effects. Transplantation. 2008; 86:36–45.

30. Hadjiyanni I, Baggio LL, Poussier P, Drucker DJ. Exendin-4 modulates diabetes onset in nonobese diabetic mice. Endocrinology. 2008; 149:1338–1349.

31. Xue S, Wasserfall CH, Parker M, Brusko TM, McGrail S, McGrail K, Moore M, Campbell-Thompson M, Schatz DA, Atkinson MA, Haller MJ. Exendin-4 therapy in NOD mice with new-onset diabetes increases regulatory T cell frequency. Ann N Y Acad Sci. 2008; 1150:152–156.

32. Sherry NA, Chen W, Kushner JA, Glandt M, Tang Q, Tsai S, Santamaria P, Bluestone JA, Brillantes AM, Herold KC. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007; 148:5136–5144.
33. Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Ealcone M, Secchi A, Bonifacio E. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand auto-reactive memory T cells. J Clin Invest. 2008; 118:1806–1814.

34. Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simoes BP, Martinez EZ, Foss MC, Burt RK, Voltarelli JC. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009; 301:1573–1579.

35. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131:861–872.

36. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin , Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318:1917–1920.

37. Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008; 283:31601–31607.

38. Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Bar-shack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000; 6:568–572.

39. Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003; 9:596–603.

40. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008; 455:627–632.
41. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004; 429:41–46.
42. Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008; 132:197–207.
43. von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007; 7:988–994.

44. Akerblom HK, Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, Teramo K, Hamalainen AM, Paronen J, Riikjarv MA, Ormisson A, Ludvigsson J, Dosch HM, Hakulinen T, Knip M. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005; 48:829–387.

45. TRIGR Study Group. Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR). Pediatr Diabetes. 2007; 8:117–137.
46. Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, Couper JJ, Colman PG. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004; 27:2348–2355.
47. Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, Manca Bitti ML, Matteoli MC, Marietti G, Ferrazzoli F, Cassone Faldetta MR, Giordano C, Sbriglia M, Sarugeri E, Ghirlanda G. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000; 43:1000–1004.
48. Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, Lecomte P, Marechaud R, Bougneres P, Charbonnel B, Sai P. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000; 356:545–549.
49. Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Getelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002; 346:1692–1698.

50. Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pioeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005; 352:2598–2608.

Figure 1.
Overview of the natural history of T1D and intervention trials according to the disease stage. Also shown are the proposed roles for innate immunity in triggering of autoimmune diabetes. Accumulation of damaged β-cells can stimulate APCs via TLR2, thus allowing them to prime diabetogenic T cells in pancreatic lymph nodes (PLN). Once sensitized, autoreactive T cells may migrate to the PLNs and induce β-cell apoptosis by direct cytolysis or indirectly via soluble mediators such as IFN-γ, TNF-α or IL-1β. In IFN-γ/TNF-α synergism model for β-cell apoptosis, CD4+T cells act in collaboration with macrophages to induce β-cell death. NF-KB is activated by IL-1β or TNF-α. When activated by IL-1β, NF-KB plays a proapoptotic role by producing nitric oxide. In contrast, NF-KB activation by TNF-α plays an antiapoptotic role by inducing antiapoptotic molecules such as XIAP that inhibits caspases. STAT1 activated by IFN-γ inhibits translation of antiapoptotic proteins.
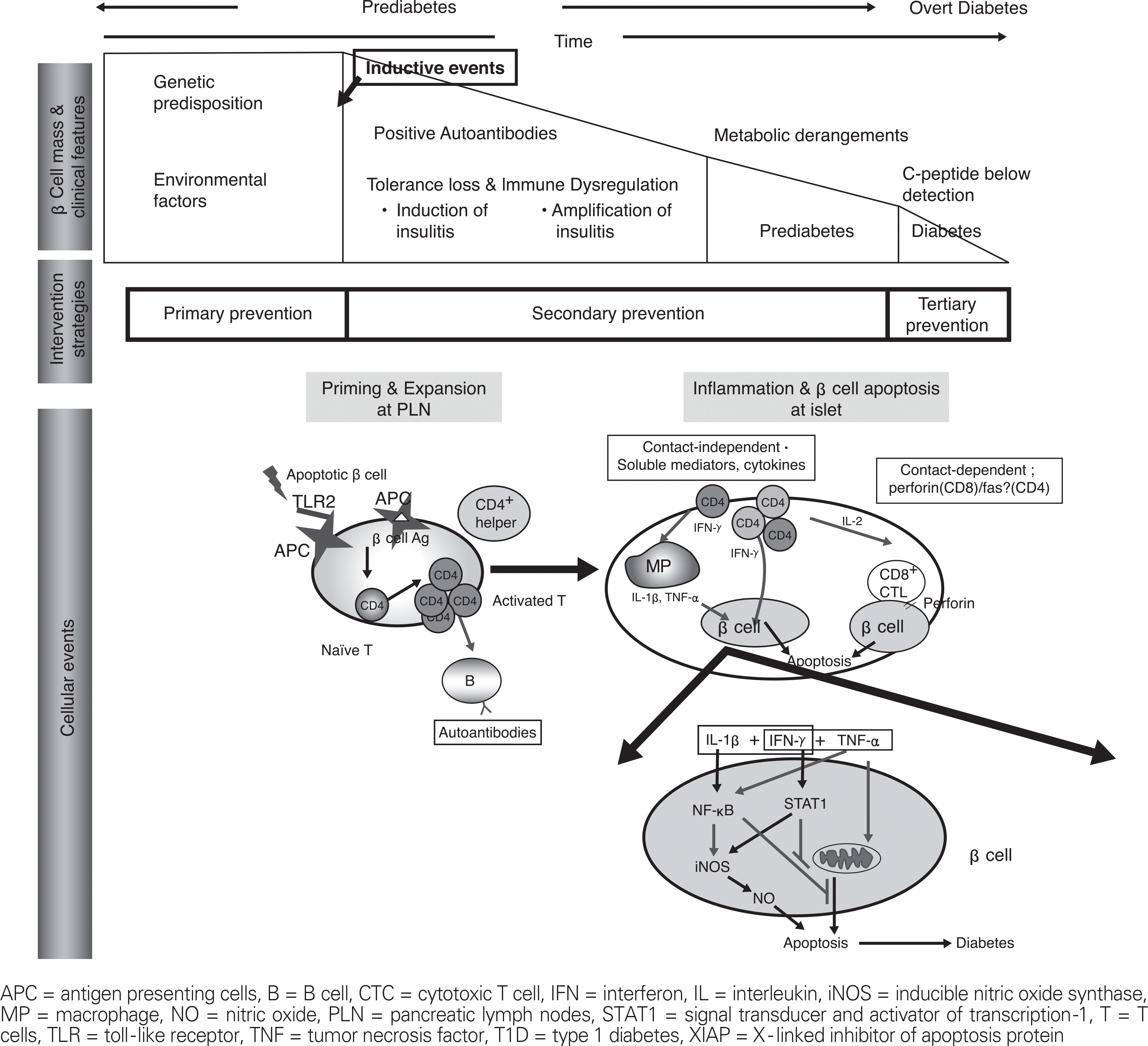
Table 1.
Representative clinical trials for prevention of T1D
| Agent/ Route | Study design | Results | Ref. |
|---|---|---|---|
| Primary Prevention: | To block autoimmunity | (at genetic risk) | |
| TRIGR (Hydrolysed cow's milk)/Oral | – Test whether delayed exposure to intact food proteins will reduce the chances of developing T1D | Ongoing |
(44) (45) |
| Oral insulin/Oral, Pre-POINT/ Intranasal insulin | – Dose finding in children with high genetic risk for T1D (Based on the beneficial effect of oral insulin (22)) | Ongoing | (19, 20) |
| Secondary Prevention: | To block /S-cell destruction | (in prediabetes) | |
| Insulin (DPT-1)/Parenteral (s.c. & i.v.) | – Low dose insulin injections in high-risk (50% over 5 years) 1st-degree relatives of T1D patients | – No effect seen on disease prevention or delay the onset of disease | (21) |
|
–Screened 84,228 relatives for ICA – 3,152 ICA-positive – 372 relatives: risk projection for T1D > 50%(parenteral insulin group) – 388 relatives: risk projection for T1D of 26∼50% over 5 years (oral insulin group) |
|||
| Insulin (DPT-1)/Oral insulin capsule |
– In relatives at intermediate risk of T1D (ICA-and IAA-positive relatives with 25∼50% 5-year risk). - Based on the concept of oral tolerance |
– No beneficial effect seen on disease progression - Post-hoc analysis; subgroup with confirmed IAA > 80 nU/mL showed a delay of diabetes of more than 4 years |
(22) |
|
The European Nicotinamide Diabetes Intervention Trial (ENDIT)/Oral |
– ICA-positive, 1st-degree relatives of individuals with T1D (projected 5-year risk of T1D was 40%). st - Screened 35,000 1st-degree relatives to identify eligible subjects. - Randomized 552 subjects |
– No effect – 4 years of follow-up, 159 subjects developed T1D (82 in the nicotinamide group and 77 in the placebo group: P = 0.97). |
(23) |
|
Insulin (INIT I)/ Intranasal |
– Older antibody-positive relatives |
– No acceleration of loss of beta cell function - Show a skewing of the immune response consistent with tolerance induction |
(46) |
| GAD-Alum Prevention Study | – Nondiabetic relatives of patients with T1D, age 3∼45, who are positive for GADA but not IAA | Ongoing | (20) |
| Tertiary Prevention: | To preserve S-cell function | (in overt T1D) | |
|
Insulin (DPT-1)/Oral Anti-CD3 mAb* (hOKT3r1 (Ala-Ala))/ i.v. |
– Recent diabetes - At onset of type 1 diabetes (within 6 weeks of T1D diagnosis) - Age 8∼30, n= 24 or 42 |
– No difference in HbA1c, C peptide, insulin requirements - Significant preservation of C-peptide secretion for at least 1 year - Reduced insulin requirement & HbA1c |
(47, 48) (17, 49) |
|
Anti-CD3 mAb (ChAglyCD3 (TRX4))/ i.v. |
– Newly diagnosed T1D - Age 12∼39, n=80 |
– Reduced insulin requirement out to 18 months (strongest effects in those with the greatest residual S-cell function at study entry) | (50) |
| rhGAD65 (alum)/s.c |
– T1D and positive GADA (within 18 months of T1D diagnosis) - Age 10∼18, n=70 |
– After 15 months, preservation of residual insulin secretion, but did not change the insulin requirement | (18) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download