Abstract
The study of pain has recently received much attention, especially in understanding its neurophysiology by using new brain imaging techniques, such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), both of which allow us to visualize brain function in vivo. Also the new brain imaging devices allow us to evaluate the patient's pain status and plan to treat patients objectively. Functional activation of brain regions are thought to be reflected by increases in the regional cerebral blood flow in the brain imaging studies. Regional cerebral blood flow increases to noxious stimuli are observed in second somatic (SII) and insular regions and in the anterior cingulate cortex and with slightly less consistency in the first somatic area (S1), motor area, supplementary motor area, prefrontal area, amygdala and contralateral thalamus. These data suggest that pain has multidimensions such as sensory-discrimitive, motivational-affective and cognitive-evaluative.
Figures and Tables
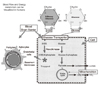
Figure 1
Putative mechanisms involved in neurometabolic and neurovascular coupling during neuronal activation.

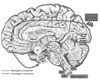
Figure 6
Putative pain pathway in the brain.
PFC: Prefrontal cortex, AMG: Amygdala, rACC: rostral Anterior cyngulate gyrus, dACC: dorsal Anterior cingulate gyrus, cACC: caudal Anterior cingulate gyrus, S1: Sensory area 1, S2: Sensory area 2, SMA: Supplementary motor area, HT: Hypothalamus, STT: Spinothalamic tract, SRT: Spinoreticular tract, SMT: Spinomesencephalic tract.
References
1. Apkarian AV, Shi T. Ayrapetian A, Apkarian AV, editors. Thalamocortical connections of the cingulate and insula in relation to nociceptive inputs to the cortex. Pain Mechanisms and Management, Series. 1997. Amsterdam: IOS Press;212–220.
2. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000. 4:215–222.

3. Wall PD, Melzack R. Wall PD, Melzack R, editors. Introduction to Pain. Textbook of pain, 4th ed Series. 1999. Edinburgh: Churchill Livingstone;1–8.
4. Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002. 3:142–151.

5. Jones AK, Brown WD, Friston KJ, Qi LY, Frackowiak RS. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc. R. Soc. Lond., Ser. B: Biol Sci. 1991. 244:39–44.

6. Josephs O, Turner R, Friston K. Event-related fMRI. Hum Brain Mapp. 1997. 5:243–248.4.
7. Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002. 125:310–319.

8. Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med. 1999. 41:1044–1057.

9. Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994. 71:802–807.

10. Cho ZH, Chung SC, Jones JP, Park JB, Park HJ, Lee HJ, Wong EK, Min BI. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci USA. 1998. 95:2670–2673.

11. Cho ZH, Chung SC, Lim DW, Wong EK. Effects of the acoustic noise of the gradient systems on fMRI-a study on auditory, motor, and visual cortices. Magn Reson Med. 1998. 39:331–335.

12. Bonvento Gilles, Sibson Nicola, Pellerin Luc. Does glutamate image your thought? Trends in neurosciences. 2002. 25:359–364.
13. Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc London B Biol. Sci. 1999. 354:1155–1163.

15. Cho ZH, Son YD, Kang CK, Han JY, Wong EK, Kim KH, Yim YK, Bai SJ, Lee UJ, Sung KK, Kim KW. Pain dynamics observed by fMRI: differential regression analysis technique. J Magn Reson Imaging. 2003. 18:273–283.

16. Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-Imaging a shared neuronal network. Science. 2002. 295:1737–1740.

17. Posner MI, Raichle ME. The neuroimaging of human brain function. Proc Natl Acad Sci. 1998. 95:763–764.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download