Abstract
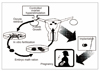
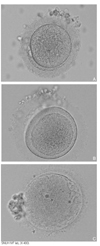
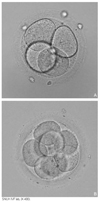
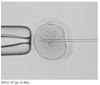
Rapid progress has been made in the field of infertility since the first IVF (in vitro fertilization) baby was born in 1978. Controlled ovarian stimulation with FSH is currently the standard procedure for ovarian stimulation before follicular aspiration. Gonadotropin-releasing hormone agonists and antagonists have been used to prevent endogenous LH surge during controlled ovarian hyperstimulation.The goal of controlled ovarian stimulation with gonadotropins is to obtain a large number of mature oocytes and thereby improve the likelihood of obtaining an adequate number of embryos for subsequent transfer. IVF was initially presented as a treatment for tubal factor infertility but was quickly utilized in other areas in the field of infertility, such as male factor infertility and even ovarian failure. ICSI (intracytoplasmic sperm injection) is a more recent approach for male factor treatment, which allows the sperm to be directly injected into the egg using micromanipulation. Preimplantation genetic diagnosis can be performed on embryos prior to the embryo transfer. The complications associated with the IVF program include ovarian hyperstimulation syndrome and multiple pregnancies. The multiple pregnancies are directly related to the practice of transferring multiple embryos at embryo transfer. Each IVF clinic publishes its pregnancy rates. However, comparisons between clinics are difficult because the success rates vary depending on the distribution of underlying causes and age of the patients. The current take-home-baby rate is only 34.7%. In 2005, the Korean government enacted a law to regulate many aspects of IVF practice.
Figures and Tables
References
2. Hendriks DJ, Mol BW, Bancsi LF, Te Velde ER, Broekmans FJ. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a metaanalysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005. 83:291–301.

3. Bancsi LF, Broekmans FJ, Mol BW, Habbema JD, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003. 79:1091–1100.

4. Oehninger S, Gosden RG. Should ICSI be the treatment of choice for all cases of in-vitro conception? No, not in light of the scientific data. Hum Reprod. 2002. 17:2237–2242.

5. Sharara FI, Queenan JT Jr, Springer RS, Marut EL, Scoccia B, Scommegna A. Elevated serum Chlamydia trachomatis IgG antibodies. What do they mean for IVF pregnancy rates and loss? J Reprod Med. 1997. 42:281–286.
6. Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980. 87:737–756.

7. Ubaldi F, Rienzi L, Ferrero S, Baroni E, Iacobelli M, Sapienza F, Cobellis L, Romano S, Scarselli F, Greco E. Natural in vitro fertilization cycles. Ann N Y Acad Sci. 2004. 1034:245–251.
8. Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance-United States, 2003. MMWR Surveill Summ. 2006. 55:1–22.
9. Van Steirteghem AC, Liu J, Joris H, Nagy Z, Janssenswillen C, Tournaye H, Derde MP, Van Assche E, Derroev P. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum Reprod. 1993. 8:1055–1060.

10. Hamberger L, Lundin K, Sjogren A, Soderlund B. Indications for intracytoplasmic sperm injection. Hum Reprod. 1998. 13:S1. 128–133.

11. Kashyap S, Moher D, Fung MF, Rosenwaks Z. Assisted reproductive technology and the incidence of ovarian cancer: a meta-analysis. Obstet Gynecol. 2004. 103(8):785–794.





 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download