Abstract
Clinical photodynamic therapy was introduced in the 1970s and has been shown to be an effective treatment modality in a variety of fields in oncology. Photodynamic therapy (PDT) is a local therapy such as radiation and surgery, which involves the photosensitization of neoplastic cells and tissues with porphyrins or related structures that catalyze, upon irradiation by laser, formation of reactive oxygen species. A photosensitizing agent is administered to the patient and after a period of 24-72 h when the concentration of the photosensitizer is maximized in tumor tissue compared with normal tissue, and then the neoplastic mass is exposed to the laser light, which initiates the necrotic process. Despite the ability of PDT to destroy the tumor selectively, it has not been applied widely due to the lack of understanding of its therapeutic mechanism and clinical experiences as well as some limitations of currently available photosensitizers. Nowadays, the number of scientific articles on PDT, regarding clinical applications as well as basic science, made its application increasing. In one of the most suitable indications, lung cancer, PDT is a minimally invasive therapeutic option for the treatment of early cancer in airway and palliation for the endobronchial obstruction from central lung cancer. In esophageal cancer, PDT can also be applied to treat in early stage without muscle invasion or remnant cancer after endoscopic mucosal resection. Besides, PDT can be applied as a part of combined modality such as a neoadjuvant or adjuvant PDT. With the advances of new sensitizers and energy delivery system, clinical application of PDT will expand in near future. This review article will focus on the basic mechanism and the clinical investigations of PDT for the clinicians.
Figures and Tables
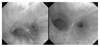
Figure 3
Bronchoscopic finding of the distal tracheal cancer just above the carina one year after left upper sleeve lobectomy (left).
Bronchoscopic finding shows disappearance of the tumor with normal mucosa 6 months after PDT (right).
References
1. Finsen NR. Phototherapy. 1901. London: Edward Arnold.
3. Ackroyd R, Kelty C, Brown N, Reed M. The history of photodetection and photodynamic therapy. Photochem Photobiol. 2001. 74:656–669.

4. Dougherty T, Kaufman J, Goldfarb A, Weishaupt K, Boyle D. Photodynamic therapy for the treatment of malignant tumors. Cancer Resear. 1978. 38:143–151.
5. Hayata Y, Kato H, Konaka C, Ono J, Takizawa N. Hematoporphyrin derivative andlaser photoradiation in the treatment of lung cancer. Chest. 1982. 81:269–277.

6. Schaffer M, Sroka R, Fuchs C, Schrader-Reichardt U, Schaffer PM, Busch M, Dühmke E. Biomodulative effects induced by 805 nm laser light irradiation of normal and tumor cells. J Phtochem Photobiol. 1997. 40:253–257.

7. Maegawa Y, Itoh T, Hosokawa T, Yaegashi K, Nishi M. Effects of near-infrared low-level laser irradiation on microcirculation. Lasers Surg Med. 2000. 27:427–437.

8. Dennis ED, Fukumura D, Rakesh KJ. Photodynamic therapy for cancer. Nature Review. 2003. 3:380–387.

9. Dougherty TJ. An Update on photodynamic therapy application. J Clinical Laser Med & Surg. 2002. 20:3–7.
10. Peng Q, Moan J, Nesland JM. Correlation of subcellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastuct Pathol. 1996. 20:109–129.

11. Fingar VH, Taber SW, Haydon PS, Harrison LT, Kempf SJ, Wieman TJ. Vascular damage after photodynamic therapy of solid tumors: a view and comparison of effect in pre-clinical and clinical models at the University of Louisville. In Vivo. 2000. 14:93–100.
12. Xue L, He J, Olenick NL. Promotion of photodynamic therapy-induced apoptosis by stress kinases. Cell Death Differ. 1999. 6:855–864.

14. Gold MH. Aminolevulinic acid photodynamic therapy: Medical evidence for its expanded use. Expert Rev Med Devices. 2006. 3:357–371.

15. Kim BM, Lim HS. Biomedical Optics and Laser treatment. Optical Science and Technolog. 2003. 7:6–13.
16. Stringer MR, Kelty CJ, Ackroyd R, Brown SB. Light dosimetry measurements during ALA-PDT of Barrett's esophagus. Photodiagnosis and Photodynamic Therapy. 2006. 3:19–26.

17. Maziak DE, Markman BR, MacKay JA, Evans WK. Photodynamic therapy in non-small cell lung cancer: A systematic. Ann Thorac Surg. 2004. 77:1484–1491.
18. McBride G. Studies expand potential uses of photodynamic therapy. J Natl Cancer Inst. 2002. 94:1740–1742.

19. Wiedmann MW, Caca K. General principles of photodynamic therapy (PDT) and gastrointestinal applications. Curr Pharm Biotechnol. 2004. 5:397–408.

20. Yoon SH, Han KT, Kim KN, Lee SI. Effect of photodynamic therapy in lung cancer. Tuberc Respir Dis. 2004. 57:358–363.

21. Jheon . Photodynamic Therapy. 2007. Seoul: Korea Medical Book Publisher Co.;89–97.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download