Abstract
The present study was performed to assess the fertility of frozen-thawed dog semen prepared by freezing with 6% glycerol and thawing at 70℃ for 8 sec, and to evaluate the least number of post-thaw spermatozoa necessary to achieve pregnancy by intrauterine or intratubal artificial insemination. It was found that the pregnancy rate of intrauterine artificial insemination was 100% using 6% glycerol buffer and thawing at 70℃ for 8 sec with 5 × 107 spermatozoa. Even though the pregnancy rate (80%) and the whelping rate (24.5%) in the 5 × 106 spermatozoa inseminated group were lower than those of the 5 × 107 spermatozoa group, conception was confirmed with 5 × 106 spermatozoa. Although the pregnancy rate of intratubal insemination was low (20%) with 4 × 106 spermatozoa, this study is the first report to show the pregnancy rate of intratubal insemination with frozen-thawed ejaculated canine semen. In order to improve the pregnancy rate with intratubal insemination of canine spermatozoa, it is necessary to investigate the optimal insemination site of the uterine tube, the appropriate number of sperm, and the direct effect of buffer on oocytes.
Artificial insemination is considered to be an in vivo assay of spermatozoa survival and is the most definitive test of sperm function. Artificial insemination with fresh or frozen-thawed ejaculated spermatozoa has been used widely and successfully in canines [4,5,11,12,15,18]. Various methods of insemination are presently available to the inseminator, including intrauterine insemination with laparotomy [6,16], the Norwegian catheter [1], fiber optic endoscopy [2], or intravaginal insemination [4,5,15,20].
Tsutsui et al. [19] reported that when using vaginally inseminated frozen semen, at least 20 × 108 motile spermatozoa were needed for fertility to be comparable to that obtained by natural mating. For frozen-thawed semen deposited into the uterus, an insemination dose of around 1.5-2 × 108 motile spermatozoa was recommended, and the rates of pregnancy ranged from 20-90% [5,14,20], although pregnancies have been obtained with lower sperm numbers [13,22]. Optimal freezing-thawing processes for dog semen will have to yield a maximal number of insemination doses from an ejaculate, but the minimum number of normal motile spermatozoa required for optimal fertility has not been studied enough in dogs.
Hammerstedt et al. [7] reported that damage to dog sperm during freezing can be reduced considerably by using the correct cryoprotectant and using it at the correct concentration. For spermatozoa of most species, glycerol has been the most commonly used cryoprotectant, although dimethyl sulfoxide has been used alone or in combination with glycerol [8]. In dogs, the glycerol concentrations used for freezing have varied between 4 and 11% (v/v) depending on the diluent composition [3]. In terms of the thawing temperature, thawing at 70℃ for 8 sec showed a significantly better survival rate of freezing spermatozoa as compared with a thawing temperature of 37℃ for 15 sec, because this required longer membrane stability [17].
The aim of the present study was to assess the fertility of frozen-thawed dog semen prepared by freezing with 6% glycerol and thawing at 70℃ for 8 sec, and to evaluate the least number of post-thaw spermatozoa needed to achieve pregnancy by intrauterine or intratubal artificial insemination. In order to exclude individual differences, semen that was frozen-thawed by the method that showed the highest motility and velocity of spermatozoa was used from one dog after testing in our preliminary study.
One male mongrel and 30 bitches from 1 to 3 years old were used as the semen donor and recipient dogs, respectively. The dogs were cared for in facilities and using procedures which exceeded the standards established by the Seoul National University for Accreditation of Laboratory Animal Care. The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals at Seoul National University, Korea.
Semen was collected once a week from a stud by digital manipulation for 8 weeks. For this procedure, a modified disposable artificial vagina was placed over the protruding penis. At the start of ejaculation, some drops of the first fraction were discarded, and only the most concentrated fraction of spermatozoa was collected. Collected semen was preserved at 37℃ in a thermos. Semen with sperm motility 80% and total ejaculated sperm ≥ 200×106 sperm/ml were used for the experiment after conventional evaluation by light microscopy using a Makler counting chamber (ZDL, USA).
Tris buffer contained Tris, citric acid, glucose, Nabenzylpenicillin, and streptomycin sulphate in 100 ml of distilled water (pH 6.5, 249 mOsm). The first freezing buffer (1st freezing extender) was made of Tris buffer with 20% (v/v) egg-yolk (pH 6.4, 843 mOsm). The second freezing buffer (2nd freezing extender) was made of the 1st extender with 12% (v/v) glycerol (pH 6.4) [3] (Table 1).
The collected semen was centrifuged at 750 × g for 5 min, and the supernatant was removed. The resultant sperm pellet was re-suspended and diluted with the first extender at a concentration of 400 × 106 sperm/ml at room temperature. The extended semen was immediately placed in a cold room at 5℃ for 1 h. Semen was then divided into 5 equal aliquots. The second dilution was performed 1h after initial dilution with the 2nd extender (12% glycerol) at 5℃. The final concentrations of glycerol were 6%, and the final semen concentration was 200 × 106 sperm/ml. After equilibration, the extended semen was loaded into 0.5 ml straws, which were sealed using an air bubble and a plastic plug, and horizontally exposed to nitrogen vapor (6 cm above the surface of the liquid nitrogen) for 10 min. Finally, straws were rapidly plunged into the liquid nitrogen (-196℃) and stored in an LN2 tank for 3 weeks until thawing.
Straws were thawed immediately prior to insemination at 70℃ for 8 sec. Thawed semen was placed in a water bath at 37℃ until insemination was performed [17].
Spermatozoa movement patterns were assessed with a computer-assisted sperm motility analyzer (CASA; Medical Supply, Korea), and sperm motility was evaluated using a sperm image analysis system (SIAS; Medical Supply, Korea), a microscope (Olympus, Japan), and a CCD camera (Toshiba, Japan). For the analysis, 10 µl of semen was placed on a pre-warmed Makler counting chamber on a plate held at 37℃.
Semen was thawed in 70℃ water for 8 sec and evaluated on the Makler counting chamber using SIAS. To verify the fertility of frozen-thawed semen, the total motile number of inseminated sperm for each of the 5 female dogs in the 3 groups was adjusted to 5 × 107, 5 × 106, or 5 × 105 sperm/ml. The total volume of inseminated semen was adjusted to 1 ml by dilution with Tris-citrate-glucose buffer (Table 1), and was surgically inseminated into both uterine horns. Intrauterine insemination was performed 3 days after ovulation. To determine the ovulation day in the dog, blood was collected daily from the cephalic vein starting on the 4th day after the beginning of bleeding at proestrus. The ovulation day was estimated according to the blood progesterone level. The sera from the centrifuged blood were analyzed using a Radioimmunoassay Kit (DSL-3900 ACTIVE Progesterone Coated-Tube; Diagnostic Systems, USA). The day on which the progesterone concentration initially entered the range of 4.0 to 7.5 ng/ml was regarded as the day of ovulation, as described by Lee et al. [10].
For surgical intrauterine insemination, anesthesia was induced with 0.1 mg/kg acepromazine and 6 mg/kg propofol, and general anesthesia was maintained with 2% isoflurane. The bitch was placed in dorsal recumbency. The ventral abdomen was clipped, and after routine surgical preparation, a 4-6 cm incision was made midway between the pubis and the umbilicus, through the linea alba. The uterus was elevated through the incision, and the prepared semen was divided in half and slowly injected into each uterine horn by means of a 3 ml syringe equipped with a 24 G IV catheter inserted at a 45-degree angle with the bevel of the needle up. Saline-moistened gauze was held over the injection site after the needle was withdrawn. After 1 min, the gauze was removed, the uterus was placed back into the abdomen and the abdominal incision was closed using a routine method. To avoid backflow of semen, the bitch was positioned with her rear elevated during recovery from anesthesia.
Corpora lutea were counted in the right and left ovaries by gently opening the thin part of the ovarian bursa at the time of insemination. The count was regarded as the number of ovulated ova. Pregnancies were diagnosed using a ultrasonograph (SONOACE 9900; Medison, Korea) with an attached 7.0 MHz linear probe 22 days post-insemination [10].
Semen was thawed in 70℃ water for 8 sec, and the total motile number of inseminated sperm for each of the 5 dogs in the 3 groups was adjusted to 4 × 106, 4 × 105, or 4 × 104 sperm/ml. The total volume of inseminated semen was adjusted to 20 µl after centrifugation at 750 × g (20 sec), and was diluted with Tris-citrate-glucose buffer (Table 1) and inseminated bilaterally into the uterine tubes. Determination of the insemination time was the same as in intrauterine insemination. Anesthesia and laparotomy were performed in the same manner as in intrauterine insemination. The prepared semen was divided in half and slowly injected into each uterine tube through the abdominal ostium with a 3.5 F Tom Cat catheter (Sherwood, USA) at a site approximately 4 cm deep from the abdominal ostium. The number of corpora lutea was counted in both ovaries at the time of artificial insemination. The count was regarded as the number of ovulated ova. Pregnancies were diagnosed using ultrasonography at 22 days post-insemination [10]. Newborns were counted on the day of delivery.
In this experiment, 5 bitches in each group inseminated with different numbers of spermatozoa were intrauterine inseminated using 5 × 107, 5 × 106, or 5 × 105 motile spermatozoa from frozen-thawed semen. Insemination was carried out with a 24 G IV catheter inserted bilaterally into the uterine horns.
In this experiment, 5 bitches in each group inseminated with different numbers of spermatozoa were intrauterine inseminated using 4 × 106, 4 × 105, or 4 × 104 motile spermatozoa from frozen-thawed semen. Insemination was performed with the aid of a 3.5 F Tom Cat catheter (Sherwood, USA) inserted into each uterine tube.
Using the statistical analysis system FREQ procedure (SAS, USA), data was analyzed by chi-square homogeneity test. In order to confirm the results of the homogeneity test, the data was incorporated into a log linear model using the SAS-CATMOD procedure (SAS, USA). Differences of p < 0.05 were considered to be statistically significant.
The pregnancy results for intrauterine insemination in Exp. 1 are shown in Table 4. Conceptions were obtained in 5 of 5 dogs (100%) in the 5 × 107 motile spermatozoa inseminated group, in 4 of 5 dogs (80%) in the 5 × 106 motile spermatozoa inseminated group, and zero of 5 dogs (0%) in the 5 × 105 motile spermatozoa inseminated group, respectively. The rates of whelping were significantly higher (p < 0.05) in the 5 × 107 (72.9%) and 5 × 106 (24.5%) intrauterine inseminated groups compared to the 5 × 105 inseminated group (0%). The average litter size in each group was 7.0, 2.4, and 0, respectively.
The pregnancy results for intratubal insemination in Exp. 2 are shown in Table 5. The conception rates were significantly higher (p < 0.05) in the 4 × 106 motile spermatozoa inseminated group (40%) compared to other inseminated groups. Conception occurred in the 4 × 105 and 4 × 104 motile spermatozoa inseminated groups. The oocyte fertilization rate (newborns/corpora lutea) and the average litter size in the 4 × 106 motile spermatozoa inseminated group were 18.2 % (8/44) and 1.6, respectively.
Intrauterine insemination with frozen-thawed semen by surgical method has been used routinely for artificial insemination in dogs. To exclude individual differences, semen was collected from only one dog.
In intrauterine insemination performed in this study, 5 conceptions were obtained 100% in the 5 × 107 motile spermatozoa inseminated group. And the 5 × 106 and 5 × 105 motile spermatozoa inseminated group conceived 80 and 0%, respectively. Results of intrauterine insemination obtained in the present study showed a higher conception rate using a lower number of spermatozoa compared to previous studies [5,14,19]. In the 5 × 107 inseminated group, the pregnancy rate was 100%, the ratio of the number of puppies to the number of ovulations was 100% in 3 out of 5 dogs, and the mean ratio was 72.9%. This inseminated number of spermatozoa seems to be appropriate to obtain successful conception compared with normal mating dogs (88%) [18].
Linde-Forsberg et al. [13] obtained a pregnancy rate of 57.9% using intrauterine insemination with 100~400 × 106 spermatozoa. Tsutsui et al. [20] performed intrauterine insemination with 100 × 106 spermatozoa in 10 dogs, achieving a 90% pregnancy rate. The results of the present study showed a higher pregnancy rate (100%) with a lower number of spermatozoa (5 × 107 spermatozoa).
When frozen-thawed semen was used for intratubal insemination in this study, the conception rate was 20% with 4 × 106 spermatozoa. Hori et al. [9] froze canine epididymal sperm and inseminated 1 × 108 spermatozoa (20 × 106 motile spermatozoa) bilaterally in the uterine tubes. They obtained fertilization only in one of the animals (16.7%) that was fertilized. Tsutsui et al. [21] obtained a conception rate of 75.0% and 37% in fresh semen intratubal insemination with 2 × 106 and 4 × 106 spermatozoa. Although the number of animals used in this study was low, no conceptions were obtained in dogs that received intratubal doses of 4×105 and 4 × 104 frozen-thawed spermatozoa. It is thought that more than 4 × 106 motile spermatozoa are needed to achieve a high conception rate when using frozen-thawed semen for intratubal artificial insemination, or that another detrimental factor of direct insemination into the uterine tubes might be an issue. It seems that the other detrimental factors of intratubal insemination with frozen-thawed semen result from the direct contact of the oocytes with the buffer, which contains a small quantity of cryoprotectant and centrifugation extender used in order to remove the cryoprotectant and adjust the sperm counts.
In a previous study, at least 20 × 108 motile frozen-thawed spermatozoa were needed in intravaginal insemination for a fertilization rate to be comparable to that obtained after natural mating [20]. For intrauterine insemination with frozen-thawed semen, an insemination dose of approximately 1.5-2 × 108 motile spermatozoa was recommended, and obtained a pregnancy rate varying from 20 to 90% [5,14,19]. Therefore, it could be presumed that the number of spermatozoa needed for intratubal insemination could be considerably smaller than that required for intrauterine insemination. However, a dose of 4 × 106 frozen-thawed spermatozoa was not enough to achieve successful conception in dogs. In terms of efficacy, optimal freeze-thaw processes for dog semen will have to yield a large number of insemination doses from one ejaculation. The result of this study showed that the pregnancy rate of intrauterine AI was 100% using 6% glycerol buffer and thawing at 70℃ for 8 sec with 50 × 106 spermatozoa. Even though the pregnancy rate (80%) and whelping rate (24.5%) in the 5 × 106 spermatozoa inseminated group were lower than those of the 5 × 107 spermatozoa group, conception was confirmed with 5 × 106 spermatozoa. Although the pregnancy rate of intratubal insemination was low (20%) with 4 × 106 spermatozoa, this study was the first report to indicate pregnancy rates following intratubal insemination with frozen-thawed ejaculated canine semen. A larger group of semen donors or pooled ejaculates should be used in further investigation in order to yield more representative and reliable results. Additionally, in order to improve pregnancy rates with intratubal insemination of canine spermatozoa, investigators must determine the optimal insemination site of the uterine tube, the appropriate number of sperm, and the direct effect of buffer on oocytes.
Figures and Tables
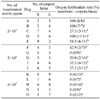
Table 2
Analysis of ejaculated fresh semen by CASA

Abbreviations: CASA, computer-assisted sperm motility analyzer; VCL, curvilinear velocity; VSL, straight line velocity; VAP, average path velocity; RAP, rapid sperm; BCF, beat-cross frequency; MAD, mean angular displacement; WOB, wobble (VAP × VCL); DNC, dance (VCL × ALH); ALH, lateral head displacement.
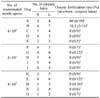
Table 3
Analysis of frozen-thawed semen post thawed at 70℃ for 8 sec

Abbreviations: Same as Table 1.
Acknowledgment
This study was financially supported by KOSEF (grant #M10625030005-06N250300510). The authors are grateful for a graduate fellowship provided by the Ministry of Education through the BK21 Program.
References
1. Andersen K. Insemination with frozen dog semen based on a new insemination technique. Zuchthygiene. 1975. 10:1–4.

2. Cremonesi F, Salamon L, Groppetti D, Pecile A. Results of a single transcervical endoscopic insemination using frozen semen in the bitch. Vet Res Commun. 2005. 29:Suppl 2. 187–189.

3. England GCW. Cryopreservation of dog semen: a review. J Reprod Fertil Suppl. 1993. 47:243–255.
4. Farstad W. Bitch fertility after natural mating and after artificial insemination with fresh or frozen semen. J Small Anim Pract. 1984. 25:561–565.

5. Farstad W, Berg KA. Factors influencing the success rate of artificial insemination with frozen semen in the dog. J Reprod Fertil Suppl. 1989. 39:289–292.
6. Ferguson JM, Renton JP, Farstad W, Douglas TA. Insemination of beagle bitches with frozen semen. J Reprod Fertil Suppl. 1989. 39:293–298.
7. Hammerstedt RH, Graham JK, Nolan JP. Cryopreservation of mammalian sperm: what we ask them to survive. J Androl. 1990. 11:73–88.
9. Hori T, Ichikawa M, Kawakami E, Tsutsui T. Artificial insemination of frozen epididymal sperm in beagle dogs. J Vet Med Sci. 2004. 66:37–41.

10. Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hossein MS, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005. 436:641.

11. Linde-Forsberg C, Forsberg M. Fertility in dogs in relation to semen quality and the time and site of insemination with fresh and frozen semen. J Reprod Fertil Suppl. 1989. 39:299–310.
12. Linde-Forsberg C, Forsberg M. Results of 527 controlled artificial inseminations in dogs. J Reprod Fertil Suppl. 1993. 47:313–323.
13. Linde-Forsberg C, Ström Holst B, Govette G. Comparison of fertility data from vaginal vs intrauterine insemination of frozen-thawed dog semen: a retrospective study. Theriogenology. 1999. 52:11–23.

14. Morton DB, Bruce SG. Semen evaluation, cryopreservation and factors relevant to the use of frozen semen in dogs. J Reprod Fertil Suppl. 1989. 39:311–316.
15. Nothling JO, Gerstenberg C, Volkmann DH. Success with intravaginal insemination of frozen-thawed dog semen-a retrospective study. J S Afr Vet Assoc. 1995. 66:49–55.
16. Olar TT, Bowen RA, Pickett BW. Influence of extender, cryoperservative and seminal processing procedures on post thaw motility of canine spermatozoa frozen in straws. Theriogenology. 1989. 31:451–461.

17. Pena A, Linde-Forsberg C. Effects of Equex, one- or two-step dilution, and two freezing and thawing rates on post-thaw survival of dog spermatozoa. Theriogenology. 2000. 54:859–875.

18. Thomassen R, Farstad W, Krogenaes A, Fougner JA, Berg KA. Artificial insemination with frozen semen in the dog: a retrospective study. J Reprod Fertil Suppl. 2001. 57:341–346.
19. Tsutsui T, Kawakami E, Murao I, Oqasa A. Transport of spermatozoa in the reproductive tract of the bitch: observations through uterine fistula. Nippon Juigaku Zasshi. 1989. 51:560–565.

20. Tsutsui T, Hase M, Tanaka A, Fujimura N, Hori T, Kawakami E. Intrauterine and intravaginal insemination with frozen canine semen using an extender consisting of orvus ES paste-supplemented egg yolk tris-fructose citrate. J Vet Med Sci. 2000. 62:603–606.

21. Tsutsui T, Hori T, Yamada A, Kirihara N, Kawakami E. Intratubal insemination with fresh semen in dogs. J Vet Med Sci. 2003. 65:659–661.

22. Wilson MS. Non-surgical intrauterine artificial insemination in bitches using frozen semen. J Reprod Fertil Suppl. 1993. 47:307–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download