Introduction
Hepatitis E virus (HEV) is an enterically transmitted causative agent of acute non-A, non-B, and icterus-inducing hepatitis in humans [21]. HEV is a nonenveloped small virus with an approximately 7.5 kb single-stranded positive sense RNA genome containing 3 open reading frames. Based on its structural and physiochemical properties, HEV was provisionally classified as part of the family Caliciviridae, genus Calicivirus. Recently, the virus was reclassified in the genus Hepevirus of the novel family Hepeviridae [2]. The transmission of the virus into a host occurs primarily by a fecal-oral route via contaminated food or water. The course of this virus in humans is self-limiting, and chronic illness is not observed [21]. Clinical signs for hepatitis E infection include jaundice, anorexia, nausea, and hepatomegaly in young adults. The disease is slightly more severe than hepatitis A, but still induces low mortality in the general population (ranging from 0.2% to 4%). In particular, hepatitis E exerts severe effects in pregnant women, with high rates of fulminating hepatitis and increases in mortality of up to 20%, particularly during the third trimester [9].
An antibody to HEV has been identified in pigs both in developing countries, such as Nepal and Thailand, and industrialized countries, including the US, Canada, Korea, Taiwan, Spain, and Australia [3,5,15,18,25,27]. A novel swine HEV isolated from a pig in Illinois in 1997 was genetically related to 2 strains of human HEV isolates identified in the US, and cross-reacted with antibodies against the human HEV capsid protein [18]. Swine HEV has since been isolated in several countries in which hepatitis E is rare, including New Zealand, Spain, the Netherlands, Japan, Canada and Taiwan [6,10,20,23,27]. Cross-species infection has been experimentally demonstrated. Specifically, the US swine HEV isolate infected rhesus monkeys and a chimpanzee, while the US-2 strain of human HEV also infected pigs [8,17]. Thus, the pig may be an animal reservoir host for HEV, and its zoonotic or xenozoonotic potential is a significant health concern. HEV infection in pigs generally occurs at 2 to 3 months of age, and approximately 80 to 100% of pigs in the US and Japan are transiently infected [4,18,20,22,]. Infected pigs are not obviously ill, and display very mild liver lesions on histopathologic examination.
Three Korean isolates of swine HEV were initially identified in sera from 2- and 3-month old pigs [4]. A serological survey revealed that approximately 14.8% of swine sera and 17.7% of human sera were positive for HEV immunoglobulin G (IgG) on an enzyme-linked immunosorbent assay (ELISA). Korean HEV isolates from humans were genetically related to Korean swine isolates and human isolates previously identified in the US and Japan [1]. Immunohistochemical methods have been applied to detect swine HEV in formalin-fixed, paraffin-embedded hepatic tissues [7].
In this study, we sought to identify swine HEV in pig liver, and detect HEV antigens in hepatic lesions of pigs from Jeju Island, using reverse transcriptase-polymerase chain reaction.
Materials and Methods
Animal samples
In total, 40 pigs from 19 farms were selected on the basis of hepatitis lesions observed when examined by microscopy. The ages of the pigs ranged from 10 to 70 days. Of these, 33 were younger than 2 months of (suckling stage), and the other 7 were older than 2 months (growing stage). Negative control liver tissues were prepared from 1-day-old colostrum-deprived pigs in Jeju Island.
Tissue processing and histopathology
All liver samples were collected from pigs at necropsy. One-third of each liver was frozen at -70℃ for polymerase chain reaction (PCR), while the remaining part was fixed in 10% neutral buffered formalin for histopathology. After fixation, samples were embedded in paraffin wax using routine procedures, cut into 3 µm sections, and stained with hematoxylin and eosin.
Reverse transcriptase PCR
Reverse transcriptase (RT)-PCR was performed to detect viral RNA in liver tissue essentially as described previously [17]. Frozen samples were washed thoroughly with physiological saline until complete removal of blood. Four foci of liver samples (1 g) were homogenized with 10 ml DNase RNase free distilled water (Invitrogen, USA). Supernatant fractions were stored at -70℃ prior to use. RNA was extracted from 200 µl aliquots of supernatants using the RNeasy Protect Mini Kit (Qiagen, Germany). All liver samples were examined by nested PCR with primers constructed from the putative capsid gene (ORF2) region, as described previously [17]. First-round PCR produced an expected fragment of 404 base pairs (bp) with the forward primer F1 (5'-AGCTCCTGTACCTGATGTTGACTC-3') and the reverse primer R1 (5'-CTACAGAGCGCCAGCCTTGA TTGC-3'). Second-round PCR, performed with the forward primer F2 (5'-GCTCACGTCATCTGTCGCTGCTGG-3') and the reverse primer R2 (5'-GGGCTGAACCAAAATCCTG ACATC-3'), produced an expected fragment of 266 bp. All PCR analyses were performed on the Thermal Cycler Dice TP600 (TaKaRa, Japan). For RT-PCR, 2 µl RNA samples were added to 18 µl one step RT-PCR mixture (Maxime RT-PCR premix; iNtRON Biotechnology, Korea). One step RT-PCR and nested PCR were performed in keeping with previously established protocols [17]. Amplified products were visualized by staining with 0.5 µl/ml ethidium bromide on a 1.2% agarose gel.
Results
Gross findings and histopathological lesions
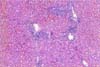
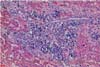
Typical gross lesions were not observed in any liver samples tested. Occasionally mild hepatic enlargement and scattered yellowish discoloration foci were observed in some samples (Fig. 1). Microscopically, mild to severe multifocal lymphoplasmacytic hepatitis was observed in 22 swine HEV PCR positive liver sections. In mild cases, we observed focal infiltration of lymphocytes, plasma cells, and macrophages in hepatic sinusoids and the portal triad (Fig. 2). Some hepatocytes and Kupffer cells were mildly swollen. Moderate to severe multifocal lymphoplasmacytic and histiocytic hepatitis or portal inflammation was noted in more severe cases (Fig. 3). In addition many oval cells were proliferated around bile ductular areas. Many hepatocytes displayed severe vacuolar degeneration and individual necrotic changes. Mild fatty change and focal hepatocellular necrosis were observed in some cases with no inflammatory reactions.
RT-PCR
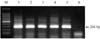
For the detection of swine HEV, RT-PCR was performed using RNA extracted from liver samples of 40 pigs. The second reaction was expected to produce a 266 bp product (Fig. 4). Swine HEV nucleic acids were detected in 22 liver samples (55%) out of the 40 pigs examined, but not in negative control pigs. Of these, swine HEV RNA was detected in 54.5% suckling pigs (18 cases) and 57.1% growing pigs (4 cases). Based on the PCR results, swine HEV positive piglets were distributed across 5 counties, Hallim (10/15), Hangyeong (1/2), Daejeong (7/15), Namwon (2/2) and Pyoseon (2/4) in Jeju Island. Seventeen cases of swine HEV positive piglets were concentrated in the counties of Hallim and Daejeong, western part of Jeju Island.
Discussion
HEV infection in pigs appears to be widespread throughout the world. Antibodies to HEV have been identified in pigs from countries where HEV is endemic, such as Nepal, China, and Thailand, and many industrialized countries, including the US, Canada, Korea, Japan, Taiwan, and Australia [3,5,10,15]. In the present study, swine HEV RNA was detected in 55% of pig livers with hepatitis lesions from different herds, indicating that infection is prevalent in different areas of Jeju Island. In a previous experiment, 3 swine HEV cases were identified out of a total of 128 pig sera collected from 10 pig herds, signifying a 2.3% rate of HEV viremia prevalence in the Korean Peninsula [4]. The overall prevalence of anti-swine HEV antibodies in pigs was approximately 14.8%. The prevalence of HEV RNA among 2-month-old domestic pigs in swine farms in various Asian countries was 2.7% in Japan [20], 1.6% in Korea [4], and 4.5% in Taiwan [26]. Considering all the different samples and methods applied, HEV infection was consistently more prevalent in pigs raised in Jeju Island than in those from the Korean Peninsula. It is evident from our data that swine HEV infection occurs at a very young age (under 2 months) in Jeju pigs. The precise natural route of HEV transmission in pigs remains to be established. Swine HEV can be transmitted experimentally via direct contact with infected pigs [16]. In fact, a recent study revealed successful experimental transmission of HEV in pigs by the fecal-to-oral route, similar to that in humans [11]. Efficient transmission of swine HEV in pigs via the fecal-oral route may require repeated exposure and high doses. Swine HEV viremia is transient, and lasts only 1 to 2 weeks, whereas fecal virus shedding may persist up to 7 weeks [8,16]. Thus, HEV in sick commercial pigs is potentially infectious to other pigs in same herds. Moreover, HEV is widespread in the general swine population in Jeju. However, naturally infected pigs did not display any clinical signs associated with HEV, although there was histopathological evidence of hepatitis, suggesting that swine HEV only caused subclinical infection in pigs in Jeju Island.
According to a previous study, various hepatitis virus antigens have been detected in liver tissue by immunohistochemical techniques [7]. However, these viral markers are only transiently expressed during the incubation and early symptomatic periods of acute viral hepatitis and may not be present during active disease, since the virus is rapidly eliminated. Therefore, immunohistochemistry is not particularly useful in the diagnosis of acute viral hepatitis, particularly since reliable serologic or molecular tests are generally available. Therefore the PCR method might be more sensitive, and thus more applicable as a specific assay for the diagnosis of various infectious diseases, compared to immunohistochemistry.
The zoonotic potential of swine HEV, based on experimental, molecular virological and epidemiological evidence, is a subject of considerable concern in many countries. All 11 pig handlers tested in China were positive for anti-HEV IgG, along with 17 (55%) of 31 apparently healthy blood donors in the same geographical region [15]. In Taiwan, 27% of the pig handlers tested positive for anti-HEV IgG, whereas only about 8% of the control subjects were seropositive [10]. In the US, the prevalence of HEV infection among North Carolina swine workers (11%) was 4.5-fold higher than that among non-swine workers (2.4%) [24]. There was a difference in anti-HEV prevalence in both swine veterinarians and blood donors among the eight selected states. Subjects from Minnesota (a major pork-producing state) were six times more likely to be anti-HEV positive than those from Alabama [19]. These findings clearly indicate that many people associated with the pig industry and researchers using pigs as experimental animals are at risk for zoonotic HEV infection. One case of hepatitis E in Korea was related to travel to a region of an HEV endemic country, India [12]. Recently, 9 cases of sporadic HEV infection were observed in Korea, without any evidence of contact with pigs [13]. Due to the relative high prevalence of swine HEV in Jeju Island, more swine practitioners and veterinarians are at increased potential risk of HEV infection within this region, compared to the Korean Peninsula.
Pigs are currently the preferable species for xenotransplantation, despite their dissimilarity to humans [14]. However, transmission of xenozoonotic pathogens from pigs to human recipient is a major problem in xenotransplantation. While swine HEV appears to be subclinical for pigs and humans in natural or experimental infections, it may become pathogenic in immunocompromised recipients of xenotransplantation. In our experiments, pigs infected with swine HEV displayed histopathological lesions of the liver. Due to the transmission of HEV from pigs to human recipients, the livers of swine HEV-infected pigs have limitations for use in xenotransplantation. Therefore, the development of a sensitive and accurate screening technology for swine HEV in donor pigs is necessary.
In summary, swine HEV nucleic acid was detected in 22 out of 40 porcine liver tissues with hepatitis. Of these, HEV was observed in 54.5% suckling pigs (18 cases) and 57.1% growing pigs (4 cases). The high prevalence of swine HEV in pigs in Jeju Island and the ability of the virus to infect across species puts individuals with swine-associated occupations at possible risk of zoonotic infection.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download