Abstract
An acidic polysaccharide of Panax ginseng (APG), so called ginsan is known to have important immunomodulatory activities. It was recently reported that APG has radioprotective effects in mice but the detailed mechanism was not fully elucidated. This study examined the effects of APG on bone marrow cells (BMs). The phenotypical and functional changes in APG-treated BMs after gamma radiation were studied. The benefit of APG on BMs damaged by gamma radiation was determined by measuring the cell viability. Using 2 different assays, a pretreatment with APG significantly increased the viability of BMs against gamma radiation. APG-treated BMs had a significantly higher amount of IL-12, which is a major cytokine for immune responses, compared with the medium-treated BMs. The expression of MHC class II molecules of APG-treated BMs was also increased, and APG-treated BMs showed significantly higher levels of allogeneic CD4+ T lymphocyte proliferation. Furthermore, APG-treated mice had a larger number of BMs after gamma radiation than the control mice, and the BMs of APG-treated mice were successfully cultured into dendritic cells, which are the representative antigen-presenting cells. Overall, this study shows that APG alters the phenotype of BMs, increases the viability and alloreactivity of BMs after gamma radiation both in vitro and in vivo. Therefore, APG may be a good candidate radioprotective agent for BMs.
Panax ginseng is a well-known medicinal herb with immunomodulatory activity. Its components include water-soluble factors, polysaccharide, protein, and saponins. An acidic polysaccharide of Panax ginseng (APG), so called ginsan is purified from the water extract of Panax ginseng using column chromatography [13]. NMR spectroscopy reveals APG to contain glucopyranoside and fructofuranoside. Since its first purification, APG has been demonstrated to have anti-tumor [13], radioprotective [20], and immunomodulatory effects including macrophage-activating activity [18].
Gamma radiation is a useful tool for anti-cancer therapy. However, there can be fatal side effects including the failure of hematopoietic system and severe immunosuppression. Therefore, the development of a radioprotective agent is expected to be an essential part of cancer therapy. Amifostine is one synthetic compound with radioprotective activity, providing free thiol in the cells, which acts as a cytoprotectant upon chemotherapy and radiotherapy [17,21]. However, clinical trials have revealed amifostine to have serious side effects [16], which might be related to its synthetic nature. In addition, there has been an emphasis on developing non-synthetic, natural product-originated radioprotective agents due to the few radioprotective agents available.
APG is a candidate therapeutic agent with radioprotective activity. It was previously reported that APG significantly enhances the number of hematopoietic and immune cells, such as bone marrow cells (BMs) and spleen cells, in irradiated mice [20]. Indeed, APG restored the reduced level of IFN-gamma [4] and modulated the antioxidant defense systems [5] in irradiated splenocytes. Thus far, the radioprotective effect of APG has been attributed to its immunomodulatory activity. Although APG increases the number of BMs in irradiated mice, it is unclear if the BMs of APG-treated irradiated mice are functional. The aim of this study was to elucidate the phenotypical and functional effects of APG. To accomplish this, this study examined whether or not a pretreatment with APG can alter the viability, phenotypical changes, cytokine production, and alloreactivity of BMs upon gamma irradiation.
C57BL/6 and Balb/c mice were purchased from Orient BIO (Korea) and maintained in the laboratory animal facility. 7- to 12 week-old female mice were used for the experiments. The animal experiments were performed in accordance with the NIH guidelines (USA) for laboratory animal use and care. APG was purified by the method as described in a previous report [13].
BMs were harvested by flushing the femurs and tibias of C57BL/6 mice. The cells were incubated with ammonium chloride-potassium carbonate (ACK) lysis buffer for 10 min to lysis red blood cells. The allogeneic CD4+ T splenocytes were prepared by mechanically disrupting spleen cells from Balb/c mice as described elsewhere [7]. The adherent cells were removed after incubating the splenocytes in RPMI 1640 media containing 10% fetal bovine serum (FBS) for 1 h. The non-adherent cells were harvested by centrifugation and used for the purification of CD4+ T lymphocytes using magnetic beads (Miltenyi Biotec, Germany) according to the manufacturer's instruction. All the cells were cultured using RPMI medium containing FBS, 2 mM L-glutamine, 100 U/ml penicillin/streptomycin (Invitrogen, USA). Bone marrow-derived dendritic cells (BMDCs) were generated using the recombinant mouse granulocyte macrophage-colony stimulating factor (GM-CSF; Biosource International, USA).
For the cell viability assays, BMs were seeded at a concentration of 1 × 106 cells/well in a 24-well culture plate, 2 × 105 cells/well in a 96-well culture plate. The cells were treated with APG for 24 h and then irradiated with 1 Gy. After 24 h, the cell viability was examined using 2 assays. First, the cells were stained with a trypan blue solution (Sigma, USA) and the number of viable and dead cells was counted using an optical microscope. Second, the cells were incubated in 96-well plate for 48 h and treated with 10 µl/well of 1 mg/ml 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma, USA) and then 10% SDS solution for 24 h subsequently. The optical density (O.D.) of the sample was measured at 570 nm using microplate reader (Molecular Devices, USA) with 630 nm as a reference.
For MLR assay, a range of cells/well of BMs of C57BL/6 mice and 1 × 105 cells/well of CD4+ T cells of Balb/c mice were co-cultured in 96-well flat-bottom culture plates (BD Biosciences, USA). The co-cultures were incubated for 5 days and pulsed with 1 µCi/well 3H-thymidine (PerkinElmer, USA). The level of thymidine incorporated was measured using a liquid scintillation counter (Wallac Microbeta TriLux; PerkinElmer, USA).
The IL-12 level was determined by ELISA using CytoSet antibody pairs (Biosource International, USA) according to the manufacturer's protocol. The level of nitric oxide (NO) was measured by harvesting the culture supernatants after incubating the BMs in a combination of APG and gamma irradiation. The cells were removed by centrifugation at 10,000 rpm for 10 sec. The nitrite levels were measured using a modified Griess reagent (Sigma, USA) according to the manufacturer's instructions. Briefly, 50 µl of the culture supernatant of the BMs was mixed with 50 µl of a modified Griess reagent at a final concentration of 40 mg/ml. The O.D. of the mixture was measured at 570 nm. A serial dilution of sodium nitrite was used as the standard.
The cells were washed with a flow cytometry staining solution, Hanks' balanced salt solution containing 5% FBS and 0.1% sodium azide, and incubated with 1 µg/100 µl each mAb for 30 min at 4℃. The cells were then stained with biotin-labeled anti-mouse I-Ab, CD86 and CD11c mAb, and sequentially with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-streptavidin (BD Biosciences, USA) for indirect staining. FITC-labeled anti-mouse I-Ab was used for direct staining. Either FITC- or PE-labeled isotype-matched mAb was used as the control. The stained cells were analyzed using a flow cytometer (FACSCaliber; Becton Dickinson, USA) and software (CellQuest; Becton Dickinson, USA).
The non-treated or APG-treated BMs were irradiated with a total dose of 1 Gy using a 60Co γ-ray source (MDS Nordion C-188 standard source installed in Applied Radiological Science Research Institute, Cheju National University, Korea).
The mice were injected with 100 mg/kg APG and after 24 h, irradiated with 5 Gy. Four days later, BMs were harvested from the femurs of mice and were counted using trypan blue exclusion test. The number of BMs was obtained from one femur of non-irradiated or irradiated mice and the mean ± SD from 4 femurs. The harvested BMs were cultured in the presence of 10 ng/ml recombinant mouse GM-CSF for 7 days. The proportion of DCs was determined by harvesting the floating cells and staining them with biotin-labeled anti-CD11c mAb, PE-streptavidin, and FITC-labeled anti-I-Ab mAb.
In MTT and MLR, the result of each sample is reported as a mean ± SD of 3 or 4 independent wells. Most of the data is representative of 3 individual experiments with similar results. The statistical significance of the data was evaluated using Tukey-Kramer multiple comparisons test (Fig. 1-4) and student's t-test (Fig. 5). *, **, *** indicate p < 0.05, 0.01, 0.001 respectively.
Trypan blue exclusion test and MTT assay were used to determine if the APG pretreatment increase the viability of BMs. In trypan blue exclusion test (Fig. 1A), the viability of the control BMs treated with the medium alone was 57 ± 3%. In contrast, the viability of the irradiated BMs was 43 ± 9%. APG significantly increased the viability of the BMs irradiated with gamma radiation. MTT assay showed that the viability of APG-treated BMs was significantly higher than the control BMs in the presence or absence of gamma irradiation (Fig. 1B). Therefore, it is believed that spontaneous cell death may have occurred in the control BMs in vitro, and APG appears to increase the viability of control BMs. Although 0.1 µg/ml APG had little effect, the cell viability was increased considerably with 1 µg/ml and 10 µg/ml APG compared with control or irradiated (IR) BMs respectively. Gamma radiation decreased the viability of BMs and APG offered significant radioprotection.
The levels of IL-12, a representative cytokine for cellular immunity, and nitric oxide (NO), a type of reactive oxygen species (ROS) with multiple functions, were measured to determine if APG alters the production of secreted cytokine and ROS. The APG treatment markedly increased the level of IL-12 production from BMs, and was not hampered by gamma radiation (Fig. 2A). However, APG did not significantly change the level of NO production from BMs regardless of the presence of gamma radiation (Fig. 2B). Since gamma radiation decreases the viability of BMs, and APG protected BMs against gamma radiation, it is unlikely that the lack of an increase in the NO levels was due to the altered viability of BMs.
The effect of APG on the phenotypical changes in BMs was examined by gating the populations of BMs according to the cell size. After acquiring the data, R1 and R2 (regions) were gated in the dot plot of each sample (Fig. 3A). R1 contains granulocytes, monocytes, macrophages, dendritic cells whereas R2 contains dead cells. Indeed, APG significantly increased the percentage of R1 from 29 ± 2% to 37 ± 2%, whereas it significantly decreased that of R2 from 23 ± 3% to 14 ± 4%. The effect of APG on the expression of major surface molecules, MHC class II and CD86, which are related to antigen-presenting capability of BMs, was next examined. APG significantly increased the level of MHC class II expression from BMs but had no effect on CD86 (Fig. 3B & C).
Bone marrow is the main source and a reservoir of hematopoietic cells, and also contains the precursor or progeny cells of antigen-presenting cells (APCs). This study evaluated the alloreactivity of BMs in a co-culture with allogeneic CD4+ T lymphocytes. APG-treated BMs strongly stimulated the proliferation of allogeneic CD4+ T lymphocytes (Fig. 4). In addition, gamma radiation did not inhibit the capability of BMs enhanced by APG. The ratio of BMs : CD4+ T lymphocytes ranged from 1 : 1 to 1 : 8, and APG-treated BMs significantly enhanced the proliferation of allogeneic CD4+ T lymphocytes at all ratios.
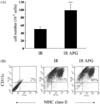
Mice were treated with APG and then with gamma radiation to determine if APG has radioprotective effects on BMs in vivo. The number of BMs harvested from APG-treated mice 4 days after gamma radiation was significantly higher than that of the control mice (Fig. 5A). In addition, the harvested BMs were cultured to generate BMDCs to determine if the BMs from APG-treated mice have a regular number of the progenitor cells of BMDCs, which is an essential antigen-presenting cell type in the immune system. Flow cytometric analysis revealed the generation of BMDCs derived from APG-treated mice to be normal (Fig. 5B).
Compounds with radioprotective activity from plant- or marine-originated materials have attracted considerable attention due to their potential use and lack of toxicity [2,9]. The compounds purified from ginseng are one group [12,22]. The ginseng root extract showed radioprotective effects on the testes of irradiated mice, no changes in the alkaline phosphatase activity and lipid peroxidation (LPO), whereas an increase in the acid phosphatase activity and LPO, and a decrease in the alkaline phosphatase activity have been observed in irradiated mice [10]. The water extract of Panax ginseng C.A. Meyer and other active components were examined for their radioprotective activity in mice based on several parameters including jejunal crypt survival and the colony formation of spleen cells [11]. One of the active components from ginseng, ginsenoside Rg3, also has a hematopoietic effect by enhancing the proliferation of bone marrow cells and splenocytes [8].
APG is an active component of ginseng, and has been shown to have immunomodulatory activity with relative safety. The drug metabolism of APG and its effects on liver have been previously examined [19]. Although there appeared to be marginal difference between males and females, APG did not show significant hepatic toxicity, and did not cause any changes in the alanine aminotransferase and aspartate aminotransferease levels. As an immunomodulator, APG significantly decreased the expression of Toll-like receptor (TLR)-2 and MyD88, an adaptor molecule for TLR signaling, which was elevated in the macrophages of mice suffering from sepsis [1]. The anti-mutagenic effect of APG on bone marrow cells was compared with those of amifostine [6]. APG reduced the number of micronucleated polychromatic erythrocytes, which are usually elevated as a result of gamma radiation.
Although APG was reported to have significant immunomodulatory and radioprotective activities [20], there is little information on the phenotypic alterations or its effect on the alloreactivity of BMs. This study showed that APG significantly increases the viability of BMs irradiated with gamma radiation using trypan blue exclusion test, MTT assay, and flow cytometric analysis. In addition, APG altered the phenotypes of BMs, which might be related to its antigen-presenting function, and increased the proliferation of allogeneic CD4+ T lymphocytes.
Gamma radiation alters the regulation of multiple genes including cytokines [14,15]. It was previously reported that some cytokines including IL-1, IL-12 stimulated the precursors of BMs and showed radioprotective and chemoprotective activity [3]. The results of our study clearly show that APG increases the level of IL-12 production. It is believed that the radioprotective effects of APG on BMs might occur through the IL-12 producing capability of APG. A molecular study of detailed mechanisms should clarify this.
APG increased the level of MHC class II expression, which is a major molecule for presenting antigenic peptides, on the surface of BMs. Furthermore, APG significantly increased the proliferation of allogeneic CD4+ T lymphocytes. This suggests that APG may enhance the immunomodulatory effects of BMs, at least in part, by increasing the surface antigen-presenting related molecules. In addition, the enhancing effect was not inhibited by gamma radiation. Interestingly, APG did not increase the level of NO production from BMs whereas it increased the level of IL-12 in the same supernatants. Although NO plays a role in the phagocytosis of macrophages, it has also inhibitory effects on the antigen-presenting capability of APCs, and acts as a potential mediator of the inflammatory process. Therefore, the lack of a significant increase in NO by APG might have both positive and negative implications for the immune responses.
Overall, APG increases the viability of BMs both in vitro and in vivo, which was not suppressed by gamma radiation. These results highlight the potential of APG as a radioprotective agent, at least for prophylactic purposes.
Figures and Tables
Fig. 1
Acidic polysaccharide of Panax ginseng (APG) increased the viability of BMs. The cells were seeded in a 24-well culture plate and treated with 10 µg/ml of APG. After irradiation, trypan blue exclusion test was performed (A). The cells were seeded in a 96-well culture plate and treated with 0-10 µg/ml of APG. After irradiation, MTT assay was performed (B). All values are represented as mean ± SD and indicated statistical significance *p < 0.05 , ***p < 0.001, respectively.

Fig. 2
APG markedly increased IL-12 production in BMs but not nitric oxide (NO). The cells were seeded and treated with APG as described in Fig. 1A. After gamma irradiation, the cells were incubated for 24 h and the supernatants were harvested. The same supernatants were used in the IL-12 (A) and NO (B) assays. All values are represented as mean ± SD and indicated statistical significance **p < 0.01, ***p < 0.001, respectively.

Fig. 3
The enhanced expression of MHC class II molecules on the surface of APG-treated BMs. BMs were seeded and treated with APG as described in Fig. 1A. The cells were stained as described in M&M. The cells acquired by flow cytometric analysis were gated (A) and a representative histogram of each sample is shown (B). The number indicates the mean fluorescence intensity, minus that of the isotype control. The mean ± SD was obtained from 3 samples (C). All values are represented as mean ± SD and indicated statistical significance ***p < 0.001.
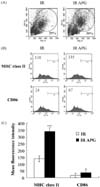
Fig. 4
APG increased the proliferation of CD4+ T lymphocytes. CD4+ T lymphocytes of Balb/c mice were co-cultured with BMs of C57BL/6 mice. After culturing for 5 days, the proliferation of CD4+ T lymphocytes was measured using 3H-thymidine incorporation assay. APG-treated BMs significantly increased the proliferation of allogeneic CD4+ T lymphocytes. The result is representative of 2 experiments. All values are represented as mean ± SD and indicated statistical significance **p < 0.01, ***p < 0.001.

Fig. 5
APG significantly increased the number of BMs and DC progenitor cells in vivo. The mice were injected with PBS alone or APG, and then irradiated. After 4 days, the number of BMs obtained from PBS-injected (control) or APG-injected mice was counted (A) and the cells were cultured in the presence of GM-CSF to verify the presence of DC progenitor cells. The number of the dot plot indicates the percentage of CD11c and MHC II double positive cells (B). Result is a representative of 2 individual experiments. All values are represented as mean ± SD and indicated statistical significance ***p < 0.001.
Acknowledgments
This research was performed under the program of Basic Atomic Energy Research Institute (BAERI) which is a part of the Nuclear R&D Programs funded by the Ministry of Science & Technology (MOST) of Korea. APG was kindly provided by Dr. Jie-Young Song (Korea Institute of Radiological and Medical Sciences, Korea).
References
1. Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006. 36:37–45.

2. Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, Prasad J, Singh S, Samanta N, Sharma RK. Radioprotection by plant products: present status and future prospects. Phytother Res. 2005. 19:1–22.

3. Dalmau SR, Freitas CS, Savino W. Radio- and chemoprotection of bone marrow cells by opposite cell cycle-acting cytokines. Leuk Res. 1997. 21:93–99.

4. Han SK, Song JY, Yun YS, Yi SY. Ginsan improved Th1 immune response inhibited by gamma radiation. Arch Pharm Res. 2005. 28:343–350.

5. Han Y, Son SJ, Akhalaia M, Platonov A, Son HJ, Lee KH, Yun YS, Song JY. Modulation of radiation-induced disturbances of antioxidant defense systems by ginsan. Evid Based Complement Alternat Med. 2005. 2:529–536.

6. Ivanova T, Han Y, Son HJ, Yun YS, Song JY. Antimutagenic effect of polysaccharide ginsan extracted from Panax ginseng. Food Chem Toxicol. 2006. 44:517–521.

7. Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001. 69:555–564.
8. Joo SS, Won TJ, Kim MS, Lee DI. Hematopoietic effect of ginsenoside Rg3 in ICR mouse primary cultures and its application to a biological response modifier. Fitoterapia. 2004. 75:337–341.

9. Kang KA, Zhang R, Lee KH, Chae S, Kim BJ, Kwak YS, Park JW, Lee NH, Hyun JW. Protective effect of triphlorethol-A from Ecklonia cava against ionizing radiation in vitro. J Radiat Res (Tokyo). 2006. 47:61–68.

10. Kumar M, Sharma MK, Saxena PS, Kumar A. Radioprotective effect of Panax ginseng on the phosphatases and lipid peroxidation level in testes of Swiss albino mice. Biol Pharm Bull. 2003. 26:308–312.

11. Lee HJ, Kim SR, Kim JC, Kang CM, Lee YS, Jo SK, Kim TH, Jang JS, Nah SY, Kim SH. In Vivo radioprotective effect of Panax ginseng C.A. Meyer and identification of active ginsenosides. Phytother Res. 2006. 20:392–395.

12. Lee TK, Johnke RM, Allison RR, O'Brien KF, Dobbs LJ Jr. Radioprotective potential of ginseng. Mutagenesis. 2005. 20:237–243.

13. Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997. 17:323–331.
14. Mori M, Desaintes C. Gene expression in response to ionizing radiation: an overview of molecular features in hematopoietic cells. J Biol Regul Homeost Agents. 2004. 18:363–371.
15. Neta R. Modulation with cytokines of radiation injury: suggested mechanisms of action. Environ Health Perspect. 1997. 105:Suppl 6. 1463–1465.

16. Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004. 70:261–264.

17. Santini V, Giles FJ. The potential of amifostine: from cytoprotectant to therapeutic agent. Haematologica. 1999. 84:1035–1042.
18. Shin JY, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002. 24:469–482.

19. Song JY, Akhalaia M, Platonov A, Kim HD, Jung IS, Han YS, Yun YS. Effects of polysaccharide ginsan from Panax ginseng on liver function. Arch Pharm Res. 2004. 27:531–538.

20. Song JY, Han SK, Bae KG, Lim DS, Son SJ, Jung IS, Yi SY, Yun YS. Radioprotective effects of ginsan, an immunomodulator. Radiat Res. 2003. 159:768–774.

21. Wrembel-Wargocka J, Jablonska H, Chomiczewski K. Clinical use of amifostine (WR-2721) as a preparation protecting healthy tissues from the cytotoxic effects of chemotherapy and radiation therapy. Przegl Lek. 1996. 53:820–825.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download