Abstract
Bisphenol A (BPA), a ubiquitous environmental contaminant, has been shown to cause developmental toxicity and carcinogenic effects. BPA may have physiological activity through estrogen receptor (ER) -α and -β, which are expressed in the central nervous system. We previously found that exposure of BPA to immature mice resulted in behavioral alternation, suggesting that overexposure of BPA could be neurotoxic. In this study, we further investigated the molecular neurotoxic mechanisms of BPA. BPA increased vulnerability (decrease of cell viability and differentiation, and increase of apoptotic cell death) of undifferentiated PC12 cells and cortical neuronal cells isolated from gestation 18 day rat embryos in a concentration-dependent manner (more than 50 µM). The ER antagonists, ICI 182,780, and tamoxifen, did not block these effects. The cell vulnerability against BPA was not significantly different in the PC12 cells overexpressing ER-α and ER-β compared with PC12 cells expressing vector alone. In addition, there was no difference observed between BPA and 17-β estradiol, a well-known agonist of ER receptor in the induction of neurotoxic responses. Further study of the mechanism showed that BPA significantly activated extracellular signal-regulated kinase (ERK) but inhibited anti-apoptotic nuclear factor kappa B (NF-κB) activation. In addition, ERK-specific inhibitor, PD 98,059, reversed BPA-induced cell death and restored NF-κB activity. This study demonstrated that exposure to BPA can cause neuronal cell death which may eventually be related with behavioral alternation in vivo. However, this neurotoxic effect may not be directly mediated through an ER receptor, as an ERK/NF-κB pathway may be more closely involved in BPA-induced neuronal toxicity.
Environmental estrogen-like chemicals have been increasingly recognized as potentially hazardous factors for human health. Some examples of this diverse group of compounds include industrial chemicals (e.g., bisphenol A, alkyl phenols, phthalates, polychlorinated biphenyls) and pesticides (e.g., DDT, methoxychlor, dieldrin, toxaphene, endosulfan). These estrogen-like chemicals are becoming ubiquitous in the environment, and humans are being exposed to these chemicals through the food chain [11]. Bisphenol A (BPA), an environmental estrogen, has been used as a compound in food and drink cans [5], as well as being a component of plastic in dental fillings. BPA has been reported to have not only estrogenic activity in the E-screen test and luciferase activity assay [31], but it also has several other biological toxicities.
Eye, nose, throat, and skin irritation and a number of cases of skin sensitization and photosensitization have been reported in man in relation to BPA toxicity. BPA also possessed immunotoxicity and reduced the non-specific host defense system [39]. In addition, recent reports demonstrated that maternal exposure to BPA affected reproductive function [41]. BPA administration during the entire period of pregnancy in rats was reported to produce pregnancy failure, pre- and post-implantation loss, fetal developmental delay, and severe maternal toxicity [18]. BPA decreased the frequency of blastocyst development in vitro [40]. However, little is known regarding the effects of BPA on the neurons, even though BPA causes embryo and developmental toxicity [18,40]. We previously found that exposure of immature mice (3-week-old) to BPA for 3 weeks resulted in neurobehavioral alteration [36].
The toxic effects of BPA have been proposed to be mediated through binding to estrogen receptor (ER)-α or -β [42]. For example, BPA reduced hepatic metallothionein synthesis and increased damage to the liver after Cd injection, and these effects occurred via an ER-mediated mechanism [38]. BPA-induced increases in uterine wet weight and in luminal epithelial height in the ovariectomized B6C3F1 mouse are mediated by ERs [30]. The ligand binding domains of ER-α and ER-β are very similar in their tertiary architecture, and many compounds bind ER-α and ER-β with similar affinities [20] or with similar potencies in activation of estrogen responsive element-mediated receptor gene expression [3]. However, there is a difference in the distributions of ER-α and ER-β [19]. The uterus, breast, pituitary, bone, and cardiovascular tissue are known to be ER-α target organs [8], whereas the ventral prostate, ovarian granulosa cells [26], and gonadotropin-releasing hormone-containing neurons in the brain [12], sympathetic ganglia [44], and immune system [37] are targets of ER-β. In addition, differential biological responses have been reported to have estrogenic compound-induced toxic effects depending on whether those chemical agents act through ER-α or ER-β [23]. However, the neurotoxic mechanism of BPA, and the relevance of its neurotoxicity to ER have not yet been studied.
Activation of the mitogen activation protein (MAP) kinase family is known to be related to cellular toxic events and numerous physiological processes such as neuronal cell death and differentiation [32]. Transcription factor, nuclear factor kappa B (NF-κB), is linked to neurite formation, as well as survival and death of neuronal cells [9]. Extracellular signal-regulated kinase (ERK) has an important temporal regulator in the form of NF-κB activation and NF-κB-dependent gene expression [16]. NF-κB also down regulates c-Jun N-terminal kinase (JNK) activation, which promotes cell death [34]. These signals have been implicated in the neurotoxic mechanisms of estrogenic environmental neurotoxic materials, unless they do not act through ER. Our previous study demonstrated that interference of differentiation of neuronal cells may be a critical factor in neuronal cell survival, and differential activation of the MAP kinase family and transcription factors are involved in survival processes [17], ochratoxin-induced neurotoxicity [27], TNF-α-induced cortical neuronal cell death [39], and Zn-induced interference of PC12 cell differentiation [35]. Therefore, in the present study, we investigated whether BPA causes PC12 cells and neuronal cell death in a dose-dependent manner, and further investigated whether the neurotoxic effects may be mediated through ER or may be related by other signals.
ICI 182,780 (Tocris, USA), Tamoxifen, PD 98,059, SB 203,580, SP 600,125, BPA, and 17-β estradiol (Sigma-Aldrich, USA) were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, USA). These chemicals were dissolved with complete medium to the desired concentrations immediately prior to use. PD 98,059, SB 203,580, SP 600,125, Tamoxifen, and ICI 182,780 pre-treatments were performed 30 min before the addition of BPA. The final concentration of DMSO was less than 0.2%.
PC12 cells that have differentiation capacity were maintained on tissue culture plastic in Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 nutrient (Invitrogen, USA) supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum, 100 µg/ml penicillin, and 100 µg/ml streptomycin at 37℃ in a 5% CO2 atmosphere. PC12 cells overexpressing ER as well as a control and PC12 cells expressing vector alone were routinely maintained in the above conditions to compare their viabilities. To induce differentiation of PC12 cells, nerve growth factor (NGF) (50 ng/ml) was added in DMEM supplied with only 1% heat-inactivated horse serum as described elsewhere [16]. Neuronal cells were prepared from E18 rat cortex (Sprague-Dawley rat brains) trypsinized (trypsin/EDTA) for 15 min at 37℃ and dissociated using a fire-polished Pasteur pipette. The resulting cell suspension was added to poly-L-lysine-coated dishes containing in neurobasal media supplemented with B 27 serum (Invitrogen, USA). The dishes were incubated at 37℃ in 5% CO2 for the designated amounts of time.
Human ER-β cDNA was produced from human testis mRNA (BD Bioscience Clontech, USA) by PCR amplification, and it was then ligated into the PGEM-T easy vector to yield a hERβ cDNA fragment (1,672 bp) containing Not I and Spe I restriction sites. Neuron specific enolase (NSE)-hERβ cDNA (6,264 bp) was obtained by ligation of the above-mentioned hERβ cDNA with NSE promoter that was cloned in PCMVβ with Not I and Spe I digestion. Human ERα cDNA (pCMV5-hERα) or NSE-hERβ cDNA was transiently transfected into PC12 cells. PC12 cells were seeded onto 96-well tissue culture plates and grown in normal growth medium. The cells were incubated until they reached approximately 50-60% confluence. We prepared solution A (200 ng ER-α or β plasmid in 100 µl serum-free DMEM) and solution B (5 µl Lipofectamine Plus reagents (Life Technology, USA) in 95 µl serum-free DMEM per well). We combined the two solutions, which were mixed and incubated at room temperature for 20 min, and then overlaid the diluted complex solution onto the cells washed once with serum-free DMEM. The medium was exchanged with normal growth medium at 4 h after transfection. The transiently ER-α and -β transfected cells were referred to as PC12/ER-α and PC12/ER-β cells, respectively. The cells stimulated in the transient transfection conditions with only vector were named PC12/neo cells.
After deleting the medium and then adding 90 µl PBS, 10 µl of WST-1 reagent (Roche, Germany) was added to each well (96-well plates). The cells were then incubated with the WST-1 reagent for 3 h in 5% CO2 at 37℃. Following incubation, the absorbance at 450 nm was determined using a microplate reader (Megellan Software; Tecan, Austria). Cell viability was expressed as the percentage of absorbance obtained in the treated wells relative to that in the untreated control wells.
Cells were cultured on 8-chamber slides. After treatment with compound for 24 h, the cells were washed with PBS and fixed with 4% paraformaldehyde at room temperature for 30 min. After fixation, dishes were washed with PBS and incubated at room temperature with 1 drop of 4,6-diamidino-2-phenylindole (DAPI) solution (Vector, USA) for 30 min in the dark. After DAPI staining, apoptotic cells were determined according to the morphological changes through fluorescence microscope (Leica Microsystems AG, Germany).
Cells were homogenized with lysis buffer: 50 mM Tris, pH 8.0, 150 mM NaCl, 0.02% sodium azide, 0.2% SDS, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 1% igepal CA 630 (Sigma-Aldrich, USA), 10 mM NaF, 0.5 mM EDTA, 0.1 mM EGTA, and 0.5% sodium deoxycholate, and were then centrifuged. Equal amounts of proteins were separated on a SDS/12% polyacrylamide gel and then transferred to a nitrocellulose membrane. Blots were blocked for 2 h with 5% (w/v) nonfat dried milk in Tris-buffered saline (10 mM Tris, pH 8.0, and 150 mM NaCl) solution containing 0.05% Tween-20. The membrane was then incubated for 3 h with specific primary antibodies, followed by incubation for 1 h with secondary antibodies. Rabbit polyclonal antibodies against ERK, p38, JNK, and mouse monoclonal antibodies against phosphorylated forms of MAP kinase (1 : 500) (Santa Cruz Biotechnology, USA) were used. Immunoreactive proteins were detected with the enhanced chemiluminescence Western blotting detection system. The relative density of the protein bands was evaluated
The gel mobility shift assay was performed using a slight modification of a method described previously [17]. In brief, the cultured cells were washed three times with ice-cold phosphate-buffered saline, pH 7.6, and pelleted. The pellets were resuspended in 400 µl of nonradioactive buffer containing 10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, and 0.2 mM phenylmetylsulfonyl fluoride, and were then centrifuged to remove everything except the nuclei. The pellets were resuspended in a second buffer containing 20 mM HEPES, 20% glycerol, 420 mM NaCl, 0.2 mM EDTA, 1.5 mM MgCl2, 0.5 mM dithiothreitol, and 0.2mM phenylmethylsulfonyl fluoride. After centrifugation, the supernatant contained the nuclear protein. The protein level was determined by a microplate modification of the Bradford method [4]. Then, 10 µg of nuclear protein was incubated in a total volume of 10 µl of binding buffer (10 mM Tris, pH 7.5, 1 mM MgCl2, 0.5 mM EDTA, 50 mM NaCl, 0.5 mM dithiothreitol, 0.05 µg/µl poly(dl-dC) • poly(dl-dC), 4% glycerol) at 4℃ for 15 min, followed by another 20 min incubation with 100 µCi of γ-32P ATP-labeled oligonucleotide containing NF-κB binding sites. The DNA protein binding complex was run a 6% nondenatured polyacrylamide gel at 150 V for 2 h. Gels were dried and autographed using Kodak MR film at -80℃ overnight. The relative density of the DNA binding bands was evaluated.
In this study, we examined the cytotoxic effects of BPA assessed by the WST-1 method. PC12 cells were cultured for 12, 24, 48, and 72 h in the condition of non-treated medium or with several doses of BPA-treated medium. The viability of cortical neuronal cells was determined after 72 h treatments with several concentrations of BPA. As seen in Fig. 1A and 2A, concentrations below 50 µM slightly increased cell viability, but at concentrations higher than 100 µM, BPA reduced the viability significantly in both types of cells. Untreated PC12 cells as well as cortical neurons contained round nuclei with homogeneous chromatin, whereas cells exposed to more than 100 µM BPA for 24 h showed a reduction in nuclear size and chromatin condensation, as well as nuclear fragmentation, all of which are typical features of apoptosis (Fig. 1B & 2B). These findings suggest that BPA can induce cell death through apoptosis. Considerable extension of neurites was observed in PC12 cells and cortical neurons after treatment with NGF (50 ng/ml) for 5 days (Fig. 1C & 2C). BPA at low concentrations (0-10 µM in PC12 and cortical neuronal cells) could increase neurite extension, but high concentrations (more than about 50 µM) reduced neurite extension.
To investigate whether damage to cells induced by BPA was mediated by ER, cells were pretreated with ER antagonists (tamoxifen, ICI 182,780) 30 min prior to treatment with BPA, and we examined PC12 and cortical neuronal cell survival using the WST-1 assay or neurite extension. ER antagonists did not abolish BPA-induced toxicity in PC12 cells, as well as cortical neuronal cells (Fig. 3A & 4A). In addition, ER antagonists also did not have significant effects on BPA-induced neurite shrinkage of the 2 types of cells (Fig. 3B & 4B). These findings seem to reflect that ER may not be related with neuronal cell damage induced by BPA.
To further confirm the involvement of ER-α or ER-β in BPA-induced neuronal cell damage, we compared the viability of PC12 using PC12 cells expressing vector alone (PC12/neo cells) and PC12 cells transfected with ER-α or ER-β cDNA after culturing with several concentrations of BPA. The toxic responses among the cells did not differ (Fig. 5B) even though PC12 cells expressing ER-α and ER-β showed 3- to 4-fold increases in ER expression (Fig. 5A).
We also compared the susceptibility of PC12 and cortical neuronal cells against BPA or 17-β estradiol using the WST-1 assay. The cell viability was determined after 72 h incubation with these chemicals. The 17-β estradiol-induced viability was similar to that induced by BPA in both cell types (Fig. 5C). This result may indicate that BPA-induced cell damages are exerted at lower levels through the classical ER-mediated intracellular receptor signal transduction system, and suggests the possibility that one or more additional signal-mediated mechanisms are more significant in BPA-induced neurotoxicity.
MAP kinase activation was determined by examining the induction of p38, JNK, and ERK, as well as their phosphorylation (p-p38, p-JNK, and p-ERK). After 2 h exposure to BPA, protein was extracted from PC12 cells and cortical neuronal cells, and was then subjected to Western analysis using each antibody that recognizes the MAP kinase family (p38, JNK, and ERK) and their phosphorylations. BPA increased the expression of p38, JNK, ERK and their phosphorylations, but only activation of ERK was clearly increased in a dose-dependent manner (Fig. 6). To study protective effects of MAP kinase inhibitions on BPA toxicities, we examined the cytotoxic effects of BPA with or without the MAP kinase inhibitors (PD 98,059, SB 203,580, and SP 600,125) assessed by the WST-1 method. Among them, only PD 98,059 (an ERK inhibitor) reversed BPA-induced toxicity of PC12 cells and neuronal cells. The other MAP kinase inhibitors did not reverse against BPA toxicities. However, it seems that the proper dose of PD 98,059 needed to protect BPA-induced neuronal damage varied. That is to say, 20 µM PD 98,059 showed the highest protective effect on PC12 cells, but a much higher dose (50-100 µM) of PD 98,059 was required to protect against BPA-induced cortical neuronal cell death (Fig. 7).
We also examined the activation of transcription factor NF-κB since it is known to act as a cell survival factor. The highest activations of NF-κB were seen in BPA (10 µM)-treated cells for 90 min. To determine the effects of BPA for NF-κB activations, PC12 and cortical neuron cells were treated with 0, 10, 50, 100, and 200 µM BPA for 90 min. The highest activations of NF-κB were seen in PC12 and cortical neuron cells treated with 10 µM BPA (Fig. 8A & B), which corresponded to the promoting effect of BPA on cell survival. However, decreases of NF-κB occurred in toxic concentrations of BPA (50-200 µM) as compared with 10 µM BPA-induced NF-κB activity. Next, we tested the effects of tamoxifen, ICI 182,780, and PD 98,059 for BPA-dependent NF-κB inactivation. PC12 and neuronal cells were pretreated with ER antagonists for 30 min and then stimulated with BPA for 90 min. As shown in Fig. 8C and D, a decrease of NF-κB activation induced by a toxic dose of BPA (100 µM) obviously was not reversed by ER antagonists in PC12 and neuronal cells. To further study the relevance of the ERK pathway in BPA-induced inactivation of NF-κB to cause a neurotoxic response, both cells were pretreated with ERK inhibitor PD 98,059 at 30 min prior to treatment with BPA, and NF-κB activity was then determined after 90 min of treatment with BPA. As expectated and in accordance with cell viability, PD 98,059 reversed BPA (100 µM)-induced NF-κB inactivation in both types of cell (Fig. 8E & F).
The present study showed that BPA reduced cell survival of PC12 and cortical neuronal cells dose-dependent manner, and this reduction was not reversed by ER antagonists. However, the susceptibility of ER-overexpressing PC12 cells was similar to that of PC12 cells overexpressing vector alone. Consistent with the dose-dependent increase of susceptibility, our experiments showed that BPA increased activation of ERK. However, the NF-κB activation level was reduced. Moreover, ERK inhibited the reversal of BPA-induced neuronal cell death and NF-κB. These results show that BPA causes neurotoxic events, and also indicated that activation of the ERK pathway accompanied with decreases of NF-κB rather than the ER pathway seems to be related to BPA-induced neurotoxicity.
The dose causing neuronal cell death in this study (more than 50 µM) is comparable to the dose inducing cell death in rat Sertoli cells [14]. However, a low dose of BPA (less than 50 µM) may aid in the neurite extension of cells, which resulted in increased cell viability. It was reported that low concentrations of BPA exert neuroprotective effects against glutamate and amyloid beta toxicities [12]. The low dose-induced cell protective effect was partially prevented in the presence of ER antagonists (data not shown), which suggests that the protective effect may be mediated by ER. However, the neurotoxic response induced by a high dose was not mediated by ER. Therefore, these data suggest that BPA has a dose-differential effect on cell survival, and a concentration of about 50 µM may be a cut-off dose to cause either protective or toxic effects on neuronal cells. Although it has been reported that BPA toxicity may be mediated by ER in other cells [43], it may not have had much influence in neuronal cell death. This notion was supported by the present data showing that ER antagonists did not significantly affect BPA-induced neurotoxicities. In addition, the toxic effect of 17-β estradiol was similar to that of BPA in PC12 cells and neuronal cells, although it was reported that the estrogenic activity of BPA is one-third to one-quarter that of estradiol [2]. Moreover, there were no significant differences in the susceptibility to BPA between the PC12 cells overexpressed with ER-α or ER-β cDNA (ER-α or β/PC12) and vector alone transfected in PC12 cells. In connection with our findings, it was reported that BPA induced marked malformations and specific apoptosis of central nervous system cells during early development of X. laevis embryos, and that these BPA effects appeared to be due to nonestrogenic activities on developmental processes [28]. Neuronal apoptosis resulting from high doses of the isoflavone genistein, known to be an estrogenic compound, also is not blocked by an ER antagonist, ICI 182,780. In their study, increased intracellular calcium and p42/44 MAP kinase were suggested as ER-independent toxic mechanisms [23]. It was also noteworthy that estrogenic response Sertoli cells incubated with nonestrogenic compounds such as 2',3',4',5'-tetrachloro-4-biphenylol and 3,3',4,4'-tetrachlorobiphenyl (estrogenic contaminants), but not with 10-7 M 17-β estradiol, showed morphological changes [33]. Moreover, a recent study showed that BPA strongly bound to ER-related receptor compared to ER-α and ER-β, which may interrupt direct binding of BPA to ER-α and ER-β [42]. Taken together, these results clearly demonstrated that BPA causes neurotoxicities, but BPA-induced neurotoxic effects may not be directly mediated by ER.
Activation of members of the MAP kinase family has been known to mediate death of several cell types, including neuronal cells [32]. Our previous data have also shown that the MAP kinase family is activated depending on the cell type and nature of stimuli in the neuronal cell survival [17,27]. Therefore, we investigated the alteration of MAP kinase signals to determine whether these signals may be related with BPA-induced neurotoxic effects. BPA activated all three types in the MAP kinase family, but only activation of ERK was clearly dose-dependent. Moreover, in the presence of ERK inhibitor, a BPA-induced neurotoxic effect was prevented, but that of other inhibitors was not, which suggested that ERK pathway activation could be involved in the neurotoxic effect of BPA. Activation of the ERK pathway as a neurotoxic mechanism has been reported on extensively. Stimulations of intracellular calcium signaling and CREB phosphorylation in mid-brain neuron death during development are associated with neurotrophin signaling by activation of MAP kinase [21]. ERK is also activated in neuronal cell death by reactive oxygen species (ROS), and its inhibition rescues cells from ROS-induced death [35]. MPTP, a neurotoxin used widely in Parkinson's disease experimental paradigms, induces activation of ERK, while its inhibition rescues the affected cells [10]. ERK inhibition also reduces toxicity following cerebral ischemia [1]. The elements leading to ERK activation include intracellular signal molecules such as thromboxane A2, intracellular calcium, angiotensin II, insulin, and ROS [24,35]. BPA-induced ERK activation may be affected by these elements in various ways. Among them, alteration of intracellular calcium concentration could be a significant contributor to the activation of ERK, thereby inhibiting cell survival. In fact, we previously found that BPA significantly lowered intracellular calcium concentrations of PC12 cells and cortical neuron cells in a dose-dependent manner [22]. In connection with the direct calcium lowering effect of BPA, estradiol inhibited ATP-induced intracellular calcium concentration in dorsal root ganglia neurons, which may modulate nociceptive signaling in the peripheral nervous system [6]. It was also reported that angiotensin-induced Ras/ERK activation is dominantly regulated by Gq-coupled Ca2+/calmodulin signaling in cardiac fibroblast cell death [25]. Therefore, it seems that the activation of ERK by depletion of intracellular calcium may be linked to the neurotoxicity of BPA.
In addition, our results demonstrated that a low concentration of BPA resulted in the development of a significant increase of the transcription factor, NF-κB, which is thought to protect cell survival. However, higher doses of BPA decreased NF-κB activity. Some evidence has accumulated to indicate that the activation of NF-κB might rescue cells from oxidative stress-induced neuronal cell death, suggesting that NF-κB activation may be an important signal to prevent cellular degeneration [29]. Based on our experiments, the transcription factors stimulated by BPA (low dose) may lead to support weak neurite outgrowths and maintainance of survival or proliferation, but high doses of BPA resulted in decreasing the effect of NF-κB, which may not be able to protect cells against BPA-induced cell death. However, the BPA-induced decrease of NF-κB activation was not reversed by ER antagonists, which again suggests that the ER pathway is not involved in BPA-induced neurotoxicity or NF-κB inactivation. In contrast, ERK inhibitor reversed a BPA-induced down-regulation of NF-κB and neuronal cell death, suggesting the involvement of the ERK pathway in the BPA-induced neurotoxic effects. These data suggests that down-activation of NF-κB may be another significant contributing factor in the BPA-induced neurotoxic mechanism.
In conclusion, BPA has a dose-dependent effect on neuronal toxicity, but the toxic effect may not be mediated by ER. The excess ERK stimulation accompanied by NF-κB inactivation is linked to BPA-induced neurotoxicity. It was reported that the normal serum BPA concentration in women is approximately 1-2 ng/ml (about 5-10 nM), and the amniotic fluid concentration of BPA is an approximately 8.5 ± 0.2 ng/ml (about 40 nM) [15]. Therefore, the concentration of BPA used in the present study was much higher (about 100-2,000 higher level) than the normal environmental exposure level. However, it is thought that the concentration of BPA in the blood could reach in the micro-molar range in the case of high-dose oral supplementation or intravenous therapy. In addition, BPA may increase physiological effects in combination with natural hormones. Thus, even though it does not waken the public interest at present, the potential for neurotoxicity of BPA needs to be considered in future studies.
Figures and Tables
Fig. 1
Effect of BPA on PC12 cell viability. (A) Cell viability of PC12 cells after 12, 24, 48, and 72 h treatments with/without several concentrations of BPA. Living cells were represented as a percentage of the total population of living and dead cells. (B) Photomicrographs of DAPI-stained PC12 cells treated for 24 h with several concentrations of BPA. Cells were stained with DAPI to visualize nuclear morphology. The percentage of apoptosis which presents a reduction in nuclear size, chromatin condensation, and nuclear fragmentation from two experiments was entered into a graph. (C) Photomicrographs of PC12 cells treated with the indicated materials for 5 days, respectively. PC12 cells were cultivated for 1 day in the condition of non-treated DMEM supplemented with Ham's F12 nutrient, 1% horse serum, 50 ng/ml NGF, 100 units/ml penicillin, and 100 µg/ml streptomycin, and were then cultured with/without several concentrations of BPA added to the medium. Control (Con), those cultured with/without BPA and NGF. *Significant difference from control (p < 0.05).
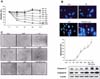
Fig. 2
Effect of BPA on cortical neuronal cell viability. (A) Cell viability of neuronal cells after 72 h treatments with/without various concentrations of BPA. Living cells were represented as a percentage of the total population of living and dead cells. (B) Photomicrographs of DAPI-stained neuronal cells treated for 24 h with various concentrations of BPA. Cells were stained with DAPI to visualize nuclear morphology. The percentage of apoptosis which presents a reduction in nuclear size, chromatin condensation, and nuclear fragmentation from two experiments was entered into a graph. (C) Photomicrographs of neuronal cells treated with BPA for 5 days, respectively. Neuronal cells were cultivated for 1 day in the condition of non-treated neurobasal medium supplemented with B 27 serum, and then cultured with/without several concentrations of BPA added to the medium. Control (Con), those cultured with/without BPA. *Significant difference from control (p < 0.05).
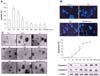
Fig. 3
BPA-induced PC12 cell death was not mainly mediated by estrogen receptor. PC12 cells were treated with ER antagonist 30 min prior to treatment with BPA. (A) Viability of PC12 cell cultures was determined after treatment with compounds for 3 days. (B) Photomicrographs of PC12 cells cultured with BPA with/without ER antagonists added to DMEM supplemented with Ham's F12 nutrient, 5% fetal bovine serum, 10% horse serum, 50 ng/ml NGF, 100 units/ml penicillin, and 100 µg/ml streptomycin for 5 days. *Significant difference from control (p < 0.05).
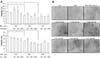
Fig. 4
BPA-induced neuronal cell death was not mainly mediated by estrogen receptor. Neurocortical (neuronal) cells were treated with ER antagonist 30 min prior to treatment with BPA. (A) Viability of neuronal cell cultures was determined after treatment with compounds for 3 days. (B) Photomicrographs of neuronal cell cultures cultured with BPA with/without ER antagonists added to neurobasal media supplemented with B 27 serum for 5 days. *Significant difference from control (p < 0.05).
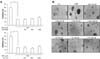
Fig. 5
BPA toxicity in PC12, PC12/neo, PC12/ER-α, or β cells. (A) Expression of ERs in established PC12 cells expressing ER-α and β. (B) Comparison of four cell types according to a viability assay performed 72 h after BPA treatments. The four cell types were cultured with/without several concentrations of BPA. (C) Comparison between BPA and 17-β estradiol on cell viability according to concentration using the WST-1 method. Viability was assayed in PC12 cells and neuronal cells treated with different concentrations of BPA and 17-β estradiol for 72 h. *Significant difference from control (p < 0.05).
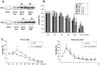
Fig. 6
Effect of BPA concentrations for activations of the MAP kinase family. Cells were treated with BPA, and after 2 h exposure to BPA, activation of MAP kinase family was determined by Western blotting. Similar patterns of expression were found from three individual experiments.

Fig. 7
Protective effects of ERK inhibitor on BPA toxicity. PC12 and neuronal cells were pretreated with MAP kinase inhibitors (PD 98,059, SB 203,580, and SP 600,125) followed by BPA (300 µM) treatment. After 72 h of exposure, we examined PC12 and neuronal cell survival using the WST-1 assay. Living cells were represented as a percentage of the total population of living and dead cells. *Significant difference from control (p < 0.05). #Significant difference from only BPA-treated group (p < 0.05).

Fig. 8
Effect of BPA, ER antagonists, and ERK inhibitor on NF-κB activation. PC12 cells (A) and cortical neuronal cells (B) were treated with 0, 10, 50, 100, and 200 µM (BPA) for 90 min with/without pretreatment of different doses of ER antagonists (tamoxifen and ICI 182,780) (C & D) or ERK inhibitor (E & F) 30 min prior to treatment with BPA. Nuclear extracts were prepared and assayed for NF-κB by EMSA from two individual experiments performed in duplicate.

Acknowledgments
This work was supported by a Korea Food and Drug Administration Research Grant, and by the Regional Research Centers Program of the Ministry of Education & Human Resources Development, Korea. We thank Dr. Carolyn L. Smith (Department of Molecular and Cellullar Biology, Baylor College of Medicine, Houston, Texas, USA) who provided human ERα cDNA (pCMV5-hERα).
References
1. Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA. 1999. 96:12866–12869.

2. Aloisi AM, Della Seta D, Rendo C, Ceccarelli I, Scaramuzzino A, Farabollini F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002. 937:1–7.

3. Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor and estrogen receptor to partial estrogen agonist/ antagonists. Mol Pharmacol. 1998. 54:105–112.

4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.

5. Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coating in food cans. Environ Health Perspect. 1995. 103:608–612.
6. Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003. 118:941–948.

7. Coleman KM, Dutertre M, El-Gharbawy A, Rowan BG, Weigel NL, Smith CL. Mechanistic differences in the activation of estrogen receptor-α (ERα)-and ERβ-dependent gene expression by cAMP signaling pathway(s). J Biol Chem. 2003. 278:12834–12845.

8. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999. 20:358–417.

9. Foehr ED, Bohuslav J, Chen LF, DeNoronha C, Geleziunas R, Lin X, O'Mahony A, Greene WC. The NF-κB-inducing kinase induces PC12 cell differentiation and prevents apoptosis. J Biol Chem. 2000. 275:34021–34024.

10. Gomez-Santos C, Ferrer I, Reiriz J, Vinals F, Barrachina M, Ambrosio S. MPP+ increases α-synuclein expression and ERK/MAP-kinase phosphorylation in human neuroblastoma SH-SY5Y cells. Brain Res. 2002. 935:32–39.
11. Guillette LJ Jr, Gross TS, Gross DA, Rooney AA, Percival HF. Gonadal steroidogenesis in vitro from juvenile alligators obtained from contaminated or control lakes. Environ Health Perspect. 1995. 103:Suppl. 31–36.

12. Gursoy E, Cardounel A, Kalimi M. The environmental estrogenic compound bisphenol A exerts estrogenic effects on mouse hippocampal (HT-22) cells: neuroprotection against glutamate and amyloid beta protein toxicity. Neurochem Int. 2001. 38:181–186.

13. Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001. 142:3261–3264.

14. Iida H, Maehara K, Doiguchi M, Mori T, Yamada F. Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod Toxicol. 2003. 17:457–464.

15. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002. 17:2839–2841.

16. Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-κB activation by ERK differentially regulates interleukin-1β-induced gene expression. J Biol Chem. 2004. 279:1323–1329.

17. Jung KM, Park KS, Oh JH, Jung SY, Yang KH, Song YS, Son DJ, Park YH, Yun YP, Lee MK, Oh KW, Hong JT. Activation of p38 mitogen-activated protein kinase and activator protein-1 during the promotion of neurite extension of PC-12 cells by 15-deoxy-Δ12,14-prostaglandin J2. Mol Pharmacol. 2003. 63:607–616.

18. Kim JC, Shin HC, Cha SW, Koh WS, Chung MK, Han SS. Evaluation of developmental toxicity in rats exposed to the environmental estrogen bisphenol A during pregnancy. Life Sci. 2001. 69:2611–2625.

19. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JÅ. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997. 138:863–870.

20. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JÅ. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998. 139:4252–4263.

21. Küppers E, Ivanova T, Karolczak M, Lazarov N, Föhr K, Beyer C. Classical and nonclassical estrogen action in the developing midbrain. Horm Behav. 2001. 40:196–202.

22. Lee YM, Lee SM, Son DJ, Lee SY, Park HJ, Nam SY, Kim DJ, Yun YW, Yoo HS, Oh KW, Kim TS, Han YS. Bisphenol a disturbs intracellular calcium homeostasis and its relationship with cytotoxicity. J Toxicol Public Health. 2004. 20:57–66.
23. Linford NJ, Yang Y, Cook DG, Dorsa DM. Neuronal apoptosis resulting from high doses of the isoflavone genistein: role for calcium and p42/44 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2001. 299:67–75.
24. Moore SA, Huang N, Hinthong O, Andres RD, Grammatopoulos TN, Weyhenmeyer JA. Human angiotensin II type-2 receptor inhibition of insulin-mediated ERK-2 activity via a G-protein coupled signaling pathway. Brain Res Mol Brain Res. 2004. 124:62–69.

25. Murasawa S, Mori Y, Nozawa Y, Masaki H, Maruyama K, Tsutsumi Y, Moriguchi Y, Shibasaki Y, Tanaka Y, Iwasaka T, Inada M, Matsubara H. Role of calcium-sensitive tyrosine kinase Pyk2/CAKβ/RAFTK in angiotensin II induced Ras/ERK signaling. Hypertension. 1998. 32:668–675.

26. Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JÅ. Mechanisms of estrogen action. Physiol Rev. 2001. 81:1535–1565.

27. Oh JH, Jung HK, Park YJ, Kim CK, Chung SY, Park NG, Yun YW, Kim DJ, Ha TY, Song YS, Lee YM, Oh K, Hong JT. Inhibitory effects of ochratoxin A on nerve growth factor-induced neurite extension through downregulation of p38 MAP kinase and AP-1 activation in cultured pheochromocytoma cells. J Toxicol Environ Health A. 2004. 67:357–371.

28. Oka T, Adati N, Shinkai T, Sakuma K, Nishimura T, Kurose K. Bisphenol A induces apoptosis in central neural cells during early development of Xenopus laevis. Biochem Biophys Res Commun. 2003. 312:877–882.

29. O'Neill LAJ, Kaltschmidt C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997. 20:252–258.
30. Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: role of the estrogen receptor. Toxicol Sci. 2000. 56:332–329.

31. Paris F, Balaguer P, Térouanne B, Servant N, Lacoste C, Cravedi JP, Nicolas JC, Sultan C. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit α and β estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002. 193:43–49.

32. Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001. 15:2508–2514.

33. Raychoudhury SS, Flowers AF, Millette CF, Finlay MF. Toxic effects of polychlorinated biphenyls on cultured rat Sertoli cells. J Androl. 2000. 21:964–973.
34. Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003. 22:3898–3909.

35. Seo SR, Chong SA, Lee SI, Sung JY, Ahn YS, Chung KC, Seo JT. Zn2+-induced ERK activation mediated by reactive oxygen species causes cell death in differentiated PC12 cells. J Neurochem. 2001. 78:600–610.

36. Seoung MJ, Shin IC, Lee YM, Son DJ, Song YS, Jeon KH, Kim YB, Lee BJ, Kim DJ, Yun YW, Kim TS, Han SY. Behavior alterations and expression of estrogen receptors in mice exposed to Bisphenol A. J Toxicol Public Health. 2004. 20:67–77.
37. Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Mäkelä S, Warner M, Gustafsson JÅ. Disruption of the estrogen receptor β gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci USA. 2003. 100:6694–6699.

38. Sogawa N, Onodera K, Sogawa CA, Mukubo Y, Fukuoka H, Oda N, Furuta H. Bisphenol A enhances cadmium toxicity through estrogen receptor. Methods Find Exp Clin Pharmacol. 2001. 23:395–399.

39. Sugita-Konishi Y, Shimura S, Nishikawa T, Sunaga F, Naito H, Suzuki Y. Effect of Bisphenol A on non-specific immunodefenses against non-pathogenic Escherichia coli. Toxicol Lett. 2003. 136:217–227.

40. Takai Y, Tsutsumi O, Ikezuki Y, Hiroi H, Osuga Y, Momoeda M, Yano T, Taketani Y. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochem Biophys Res Commun. 2000. 270:918–921.

41. Takao T, Nanamiya W, Nagano I, Asaba K, Kawabata K, Hashimoto K. Exposure with the environmental estrogen bisphenol A disrupts the male reproductive tract in young mice. Life Sci. 1999. 65:2351–2357.

42. Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol Lett. 2006. 167:95–105.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download