Hepatocellular adenoma is a benign neoplastic proliferation of hepatocytes, and it is uncommon in animals, although it has occasionally been reported in dogs, cats, sheep, pigs, primates and exotic avian species [4,10]. Although hepatocellular adenoma generally occurs in older animals, it has been described in pigs under 6 months of age and in sheep less than 1 year old [2]. In dogs, it is less frequently diagnosed than its malignant counterpart [9,15]. In humans, hepatocellular adenoma develops more frequently in women than in men [11], but no comprehensive breed and gender predisposition has been identified in animals.
Numerous cases of tumor have been reported in ferrets. In contrast, there have been a far smaller number of cases reported in otters, including hepatocellular carcinoma [16], lymphosarcoma [5], malignant seminoma [12], malignant ovarian teratoma [17], concurrent cholangiocellular carcinoma, leiomyoma, pheochromocytoma [14] and differentiated basal cell carcinoma [8]. We describe here a spontaneous hepatocellular adenoma with exocrine pancreatic nodular hyperplasia in a Eurasian otter (Lutra lutra) in Korea.
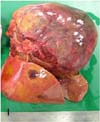
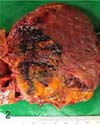
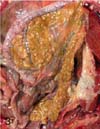
A 7-year-old female Eurasian otter, which was kept in an outdoor exhibit at the Seoul Grand Park, Korea, died suddenly after several days of displaying depression, anorexia, weight loss and rough skin. At necropsy, a solitary friable, mottled, dark red to yellow mass that was approximately 12 × 9 × 5 cm in size with a pale and dull margin was found bulging from the right hepatic lobe (Fig. 1). On cut section, the neoplastic mass had a widespread area of focal red or yellow discoloration that was associated with hemorrhage and necrosis, respectively (Fig. 2). No metastasis to the other parenchymal organs was noted. Numerous small tan foci that ranged from 0.5 to 1.0 cm in diameter were found to be evenly scattered throughout the pancreas (Fig. 3). Representative tissue samples, including the hepatic mass and pancreas, were fixed in 10% phosphate-buffered formalin, routinely processed, embedded in paraffin, sectioned at 3 µm, stained with hematoxylin and eosin and then they were examined by light microscopy.
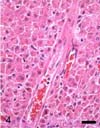
Microscopically, the neoplastic mass was non-encapsulated and poorly circumscribed, and it consisted of neoplastic hepatocytes arranged in solid sheets. There was moderate anisocytosis and anisokaryosis. The neoplastic cells had distinct borders, moderate to abundant amounts of eosinophilic cytoplasm that contained glycogen and lipid droplets, and centrally located round nuclei with one to 2 prominent nucleoli. There were 0-1 mitotic figures per high power field (× 400). The majority of portal triads were obliterated by invasion of neoplastic cells (Fig. 4), with only a few remnants of the portal triads being recognizable. Neither intrahepatic nor extrahepatic metastases were observed. In the pancreas, we observed nonencapsulated, well-demarcated foci of hyperplastic nodules of the exocrine pancreatic glands.
Gross and histologic examinations resulted in the differential diagnosis of hepatic nodular hyperplasia, hepatocellular adenoma and hepatocellular carcinoma. The presence of destroyed portal triads by neoplastic cell invasion was helpful in discriminating the current case from nodular hyperplasia. It may be difficult to distinguish between hepatocellular adenoma and well-differentiated hepatocellular carcinoma. Although a hepatocellular carcinoma may be large in size before metastasizing, indications of malignancy such as metastases to other parenchymal organs, cellular atypia and invasiveness to the normal adjacent hepatic parenchyma are useful for the differential diagnosis [3]. On the basis of the absence of these indications, a diagnosis of hepatocellular adenoma was made in the present case.
Hepatocellular tumors are caused by several factors. In humans, hepatitis B virus is an epidemiologically proven risk factor, with individuals in Asia and the sub-Saharan region having a higher probability of contact with the hepatitis virus than do those individuals in Western countries [6]. Similarly, chronic infection with hepadnavirus and Helicobacter hepaticus can lead to hepatocellular carcinoma in woodchucks [13] and mice [4], respectively. No evidence of chronic hepatitis or cirrhosis was noted in the present case. In addition to the infectious agents, genetic, dietary, aging and environmental factors are etiologically related to hepatocellular tumors in animals and humans [1,7]. In animals, however, no regional prevalence or interspecies differences in incidence have been identified due to the limited number of reported cases. In the present study, the diagnosis of hepatocellular adenoma could be established histologically in a Eurasian otter that was kept in an outdoor exhibit, but the exact etiology and pathogenesis of this disease are not known.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download