Abstract
Potential negative effects of exposure to Nigerian Qua Iboe Brent crude oil on the reproductive system of male rats was investigated. Forty Sprague-Dawley rats were used for the experiment. Exposure to Nigerian Qua Iboe Brent crude oil was achieved via oral administration of increasing doses (0.1, 0.2, and 0.4 ml/rat) every other day for 4 weeks. Cauda epididymal sperm reserves and relative weights of the testes as well as histological features of the testes of rats that received the crude oil treatment were compared to those of control rats. The results described here showed a significant (p < 0.01) dose-dependent reduction in the cauda epididymal sperm reserves of rats that received crude oil treatment relative to the control group. The morphology of testes of the crude oil-exposed rats was characterized by the presence of interstitial exudates, degeneration, and necrosis of spermatogenic and interstitial (Leydig) cells. Findings indicate that exposure of male rats to Nigerian Qua Iboe Brent crude oil may have adversely affected their reproductive systems. This may imply possible reproductive health hazards for animals and humans that may be exposed to this environmental pollutant, especially in areas where oil spillage is a common feature.
Nigerian Qua Iboe Brent crude oil is produced by ExxonMobil and is obtained from numerous off-shore fields in the Bight of Biafra in southeastern Nigeria, east of the Oso field. The API gravity for Qua Iboe is 36 degrees, and the sulfur content is 0.1%. Other specifications include: specific gravity, 0.8487; pour point, 60℉; nickel 4.1 wppm; vanadium 0.3 wppm; viscosity (20℃), 5.71 cSt.
Crude oil is an important environmental and industrial pollutant. The major chemical composition of petroleum (crude oil), its hydrocarbons tend to differ widely depending on the location and source [3]. In our environment, these chemicals are capable of mimicking the inherent actions of reproductive hormones and, hence, have the ability to disrupt the neuroendocrine system or the function of the gonads directly [28].
The increasing trend of male reproductive impairment [2,16,25] observed in some countries has been associated with possible exposures to chemicals that could interfere with endocrine homeostasis (endocrine disrupting chemicals, EDC). Some chemicals that exhibit estrogen-like activity have also been incriminated as the causative agents for cryptorchidism, testicular cancer, other testicular abnormalities, and declining sperm counts [21,25]. Chemical compounds with endocrine-mimicking characteristics such as pesticides, alkylphenols, phthalates, disinfection byproducts of water purification, common chemical contaminants, phytoestrogen, and estrogenic mycotoxins are found in packaged foods, drinking water, lakes, and oceans [4,10].
Following any oil spill, a number of simultaneous processes occur: spreading, dispersion, volatilization, evaporation, photo-oxidation, emulsification, sedimentation, and biodegradation, which together determine the fate of the constituent hydrocarbons [7,12,17]. The toxicity of a petroleum fraction is related to its hydrophobicity [6] because lipid solubility is an important factor in the passage of petroleum components through the cell plasma membrane, as well as the degree of membrane disruption.
Despite the ominous potential for catastrophic health hazards, with regards to petroleum toxicosis, there is little or no information on the long-term consequences of exposure of this environmental pollutant on the male reproductive system. The vulnerability of the reproductive system of animals to EDC exposure may be enhanced in oil spill areas. Therefore, the present study was designed to investigate the possible changes in the testicular morphology and cauda epididymal sperm reserves of male rats exposed to Nigerian Qua Iboe Brent crude oil.
The forty male Sprague-Dawley rats used for this study were obtained from the animal house of the Department of Veterinary Anatomy, University of Nigeria, Nsukka. These animals weighed between 135 to 150 g, and were housed in fly-proof metal aluminum cages and fed commercially-prepared feed ad libitum. Drinking water was also provided. The rats were held for 2 weeks for acclimatization prior to the commencement of the experiment, and were randomly assigned to 4 groups (n = 10). The control group rats received no crude oil, while rats of the test groups received Nigerian Qua Iboe Brent crude oil every other day via the oral route using a drenching tube. On each treatment day, the low dose group received 0.1 ml of crude oil per rat, the medium dose group received 0.2 ml of crude oil per rat, and the high-dose group received 0.4 ml of crude oil per rat. At the end of 4 weeks of treatment with crude oil, each rat was sacrificed by cervical dislocation The ethical guidelines for animal protection rights were observed.
Following death, the epididymides of rats in the control and test groups were dissected and extraneous tissues were trimmed. They were weighed using a Mettler balance before being put in bijour bottles containing phosphate buffered saline, pH 6.8. The cauda epididymal sperm reserves were determined using the standard hemocytometric method [1].
Specimens of the dissected testes of rats in the control and test groups were weighed. Testicular weight was assessed relative to animal live body weight (gram testicular weight per gram live body weight × 100). The specimens were then fixed by immersion in Bouin's fluid for 48 h. Later, they were dehydrated in graded levels of ethanol, cleared in xylene, and embedded in paraffin wax for sectioning. The 5 µm thick sections were cut, mounted on glass slides, and stained with hematoxylin and eosin for light microscopy.
Table 1 presents the data regarding cauda epididymal sperm reserves and relative weights of the testes of rats that received graded doses of crude oil. The cauda epididymal sperm reserve was significantly reduced in the low-dose (p < 0.05), medium-dose (p < 0.01), and high-dose (p < 0.01) groups compared to the control group. Increased dosages of crude oil resulted in dose-dependent reduction in cauda epdidymal sperm reserve. The medium dose significantly reduced the cauda epididymal sperm reserve relative to the low dose (p < 0.01). Similarly, the high dose significantly reduced the cauda epididymal sperm reserve relative to the low and medium doses (p < 0.01).
The high-dose group had a significantly increased mean relative weight of the testes when compared to the control and medium dose groups (p < 0.05). The mean relative weight of the testes was also significantly increased in the low dose group relative to the medium dose group (p < 0.05), but there were no significant differences (p > 0.05) between the relative testicular weights of the low dose and control groups.
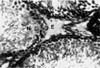
Testes taken from animals of the control group showed normal seminiferous tubules and interstices (Fig. 1). The morphology of testes of the crude oil-exposed rats was characterized by the presence of interstitial exudates, degeneration, and necrosis of spermatogenic and interstitial (Leydig) cells (Fig. 2). The magnitude of this pathology was dose-dependent. Based on visual estimation, the high dose appeared to have given rise to marked reduction in the number of spermatocytes, spermatids, and spermatozoa relative to the control (Fig. 3). An increase in the number of spermatogonia was apparent.
Spermatogenesis is the sum total of events that occur within the testis that produce spermatozoa [11]. Spermatogenesis occurs within the seminiferous tubules of the testis. It is a lengthy, sequential process by which stem cell spermatogonia divide by mitosis to maintain their own numbers and to cyclically produce primary spermatocytes that undergo meiosis to produce haploid spermatides, which differentiate (without further division) into spermatozoa. The efficiency of spermatogenesis is assessed according to the number of spermatozoa produced per gram of testicular parenchyma and is not influenced by the differences in testicular size among animals.
In this study, a significant dose-dependent reduction was observed in the cauda epididymal sperm reserves of male rats exposed to Nigerian Qua Iboe Brent crude oil. This oligospermia is an indication that the crude oil interfered with testicular spermatogenesis. The observed reduction in cauda epididymal sperm reserves of the exposed rats suggests depression of spermatogenic activity, which probably indicates a decrease in the number of developing germ cells.
Crude oil has always been identified as a potential source of polyaromatic hydrocarbons (PAHs), which have the potential to induce adverse developmental defects such as termination of pregnancy, malformations, sterility in offspring, testicular changes such as wasting with lack of sperm, immunosuppression, and tumors [14]. Oil contains PAHs, and laboratory studies have shown that exposing sexually mature salmon (Salmo salar sebago) and flounder (Pseudopleuronectes americanus) to oil generally results in decreased levels of male hormones [27], which invariably effects spermatogenesis. Additionally, if salmon are exposed to crude oil during the final stages of maturation, testicular development may be inhibited [27].
This study recorded a relative increase in testicular weight of the high-dose treatment group. Testicular size/weight alone does not determine the level of sperm production. Reduced sperm head count was not associated with decreased testicular weight in adult rats after exposure to an environmental toxicant, lindane [5].
The testicular histology of this work revealed severe degeneration and necrosis of spermatogenic cells. This was characterized by marked reduction in the number of spermatocytes, spermatids, and spermatozoa. Furthermore, there was a relative increase in the number of spermatogonia. These findings acted as an indicator that the maturation of spermatogonia through the process of meiosis has been severely disrupted following crude oil exposure. There have been reports of decreased sperm counts in the testis and epididymides of rats that were exposed to graded levels of Nigerian bonny light crude oil. These findings suggest the inhibitory action of this variety of crude oil on spermatogenesis [18]. Necrosis and disintegration of spermatocytes resulted from administration of a low dose of mercury to male mice [19]. Cyclophosphamide was found to mainly affect differentiating spermatogonia with little or no stem cell loss [13]. Again, trophosphamide administered in vivo was highly cytotoxic for mouse testis germ cells, and specifically acts on the differentiating spermatogonia compartment [24]. The death of a reasonable number of differentiating spermatogonia resulted in a reduced number of tetraploid cells (mainly spermatocytes) after 7 days and, subsequently, fewer round spermatids after 21 days and elongated spermatids after 28 days.
In this particular study, degeneration and necrosis of interstitial (Leydig) cells following exudation into the interstices was observed in the testes of rats that received crude oil treatment. Although a testosterone assay was not performed, the necrosis of the interstitial cells probably would have resulted in decreased synthesis of this hormone, which is well known to support spermatogenesis [9].
Enzymes such as lactate dehydrogenase (LDH-c4) and succinate dehydrogenase (SDH) are known to be primarily associated with postmeiotic germ cells, as shown by their increase during the growth process of the testes [15,22] and by immunohistochemistry [8]. Changes in the LDH-c4 and SDH activities associated with impairment of the spermatogenesis have been consistently induced by testicular toxicants [20,23,26]. It is possible to infer from this study that the activities of these enzymes were altered, and these altered activities may have led to spermatogenic dysfunction, as evidenced by the observed reduction in cauda epididymal sperm reserves, degeneration, and necrosis of the spermatogenic cells in the rats that were exposed to crude oil treatment.
In conclusion, the present study has demonstrated that exposure of rats to crude oil induces reproductive cytotoxicity confined to the differentiating spermatogonia compartment. Therefore, it is conceivable that crude oil has the potential to hamper not only male animal gem cell development, but also human male germ cell development. This environmental toxicant likely poses great reproductive risk to animals and humans in areas where continual oil spillage occurs.
Figures and Tables
Fig. 1
Testicular section from control rat showing seminiferous tubules (T) with normal spermatogenic cells. Note the interstitial spaces (S). H&E stain, × 400.
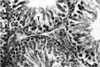
Fig. 2
Testicular section from rat exposed to the low dose of crude oil. Note exudation (E) into the interstitial space, and degeneration/necrosis (N) of spermatogenic cells. H&E stain, × 400.
References
1. Amann RP, Almquist JO. Reproductive capacity of dairy bulls. 1. Techniques for direct measurement of gonadal and extra-gonadal sperm reserves. J Dairy sci. 1961. 44:1537–1545.
2. Bergström R, Adami HO, Möhner M, Zatonski W, Storm H, Ekbom A, Tretli S, Teppo L, Akre O, Hakulinen T. Increase in testicular cancer in six European countries: a birth cohort phenomenon. J Natl Cancer Inst. 1996. 88:727–733.

3. Briggs KT, Yoshida SH, Gershwin ME. The influence of petrochemicals and stress on the immune system of seabirds. Regul Toxicol Pharmacol. 1996. 23:145–155.

4. Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993. 101:378–384.

5. Dalsenter PR, Faqi AS, Webb J, Merker HJ, Chahoud I. Reproductive toxicity and tissue concentrations of lindane in adult male rats. Hum Exp Toxicol. 1996. 15:406–410.

6. Freedman B. Environmental Ecology: The Ecological Effects of Pollution, Disturbance and Other Stresses. 1995. 2nd ed. San Diego: Academic Press;159–188.
7. Gerlach SA. Marine Pollution: Diagnosis and Therapy. 1981. Berlin: Springer-Verlag;21–46.
8. Hintz M, Goldberg E. Immunohistochemical localization of LDH-X during spermatogenesis in mouse testes. Dev Biol. 1977. 57:375–384.

9. Jewell WT, Hess RA, Miller MG. Teaticular toxicity of molinate in the rat: metabolic activation via sulfoxidation. Toxicol Appl Pharmacol. 1998. 149:159–166.

10. Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995. 103:582–587.

11. Johnson L. Cupps PT, editor. Spermatogenesis. Reproduction in Domestic Animals. 1991. 4th ed. San Diego: Academic Press;173–219.

12. Jones AM. The environmental effects of North Sea oil. Sci Prog. 1989. 73:457–468.
13. Lu CC, Meistrich ML. Cytotoxic effects of chemotherapeutic drugs on mouse testis cells. Cancer Res. 1979. 39:3575–3582.
14. Lyons G. Briefing on Polyaromatic Hydrocarbons (PAHs) Submitted by the World Wide Fund for Nature (WWF). 1998. Copenhagen: Working Group on Sea-Based Activities (SEBA);16–20.
15. Meistrich ML, Trostle PK, Frapart M, Erickson RP. Biosynthesis and localization of lactate dehydrogenase X in pachytene spermatocytes and spermatids of mouse testes. Dev Biol. 1977. 60:428–441.

16. Moller H. Trends in incidence of testicular cancer and prostate cancer in Denmark. Hum Reprod. 2001. 16:1007–1011.

17. Neff JM. Geraci JR, St. Aubin DJ, editors. Composition and fate of petroleum and spill-treating agents in the marine environment. Sea Mammals and Oil: Confronting the Risks. 1990. San Diego: Academic Press;1–32.

18. Orisakwe OE, Akumka DD, Njan AA, Afonne OJ. Testicular toxicity of Nigerian bonny light crude oil in male albino rats. Reprod Toxicol. 2004. 18:439–442.

19. Orisakwe OF, Afonne OJ, Nwobodo E, Asomugha L, Dioka CE. Low-dose mercury induces testicular damage protected by zinc in mice. Eur J Obstet Gynecol Reprod Biol. 2001. 95:92–96.

20. Pant N, Shankar R, Srivastava SP. In utero and lactational exposure of carbofuran to rats: effect on testes and sperm. Hum Exp Toxicol. 1977. 16:267–272.

21. Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993. 341:1392–1395.

22. Shen R, Lee IP. Developmental patterns of enzymes in mouse testes. J Reprod Fertil. 1976. 48:301–305.
23. Sinha N, Narayan R, Shankar R, Saxena DK. Endosulfan-induced biochemical changes in the testes of rats. Vet Hum Toxicol. 1995. 37:547–549.
24. Spanò M, Bartoleschi C, Cordelli E, Leter G, Tiveron C, Pacchierotti F. Flow cytometric assessment of trophosphamide toxicity on mouse spermatogenesis. Cytometry. 1996. 24:174–180.

25. Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ Jr, Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Muller J, Rajpert-De Neyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996. 104:741–803.

26. Traina ME, Fazzi P, Urbaniand E, Mantovani A. Testicular creatine and urinary creatine-creatinine profiles in mice after the administration of the reproductive toxicant methoxyacetic acid. Biomarkers. 1997. 2:103–110.

27. Truscott B, Walsn JM, Burton MP, Payne JF, Idler DR. Effect of acute exposure to crude petroleum and some reproductive hormones in salmon and flounder. Comp Biochem Physiol C. 1983. 75:121–130.

28. Veeramachaneni DN. Deteriorating trends in male reproduction: idiopathic or environment? Anim Reprod Sci. 2000. 60-61:121–130.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download