Abstract
The Japanese encephalitis virus (JEV) is one of causative agents of reproductive failure in pregnant sows. An indirect enzyme-linked immunosorbent assay (I-ELISA) was examined for its potential use in the rapid monitoring of the JEV, and the results were compared with those from the hemagglutination inhibition (HI) and serum neutralization (SN) tests. The comparative analysis showed that the results of I-ELISA showed a significant correlation with the conventional HI (r = 0.867) and SN tests (r = 0.804), respectively. When the I-ELISA results were compared with the traditional diagnostic assays, the sensitivity of the I-ELISA was 94.3% with the HI test and 93.7% with the SN test, respectively. The specificity was found to be 81.4% and 80.0% with the HI and SN tests, respectively. To determine the applicability of I-ELISA in the field, the serum samples from 720 pigs were collected from 4 regions in Korea between July and August 2004. The results indicated that 21.7% of screened pigs were seropositive for the JEV. The seropositive rates of JEV in the 4 provinces were 12.6% in Gyeonggi, 45.0% in Gyeongnam, 16.7% in Jeonbuk, and 12.2% in Jeju. The I-ELISA methodology developed in this study was shown to have considerable sensitivity and specificity through a comparison with HI and the SN tests. Therefore, it might be one of convenient methods for screening a large number of samples in various fields.
The Japanese encephalitis virus (JEV) is a small, enveloped virus with a single stranded positive sense RNA genome, approximately 11 kb long, containing three structural genes, nucleocapsid (C), membrane (M), envelope (E), as well as seven non-structural genes, NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5, and two non-translating regions (NTR) at both the 5' and 3' ends [1]. The JEV is classified into 4 genotypes based on the prM gene sequence [3]. Other Flaviviruses like Kunjin virus (KUN), Murray Valley encephalitis virus (MVE), St. Louse encephalitis (SLE), and West Nile virus (WN) also belong to the JEV serocomplex.
Japanese encephalitis is defined as a reproductive disorder of pigs caused by the JEV, which belongs to the genus Flavivirus of the family Flaviviridae. Although signs of the disease are not observed in adults and growing pigs, breeding pigs infected with the JEV show reproductive problems such as stillbirths and mummified fetuses. The causative agent of the disease was first identified in Korea in 1935, and was isolated from a newborn piglet in 1969 [6]. The live attenuated vaccine for swine was developed in 1980s and has been used to control the natural amplifying host. In 1999, the JEV (KV1899 strain) was isolated from pig blood in Korea [19].
Various serological tests such as the serum neutralization (SN) test and hemagglutination inhibition (HI) test [4,9,14,15], have been used to detect various JEV specific antibodies such as NS1 specific IgG, IgM, premembrane and envelope (prME) specific IgG [10,13,18]. Currently, a laboratory serodiagnosis of JEV infection is mainly carried out for the HI test to detect the polyclonal antibodies in the sera or thoracic fluid from aborted fetuses. However, the HI test has limited laboratory performance in that the inactivated whole virus as an agglutination antigen and goose red blood cells are needed, and the procedure is complicated. Furthermore, a preadjustment time is usually required to set up the standard. Although nonstructural proteins are ideal candidates for identifying antibodies against the virus specific protein that is present only in infected animals [13], this approach is not applicable in Korea because a live attenuated vaccine has been used to control the JEV in Korea. A diagnosis of the JEV is made based on isolating the virus from infected animals or the detection of serum antibodies from fetal thoracic fluids by either HI or SN tests. The reverse transcription polymerase chain reaction has been also used to detect the Flavivirus genome in various biological samples such as infected cell cultures and mosquitoes [11]. Recently, a particle agglutination assay system using hydroxyapatite-coated nylon beads was reported to be a suitable diagnostic method [18]. However, there are several difficulties in using nylon beads e.g. standardization with a limited number of samples. For this reason, a simple and convenient serological method was developed for the JEV. I-ELISA was investigated for its potential use in the serodiagnosis of the JEV-specific antibody, and the efficacy was compared with those traditional HI and SN tests.
The viral antigens for the HI test were prepared using a sucrose-acetone extraction method from the brain of a suckling mice infected with the JEV Nakayama strain [5]. The Korean strain JEV (KV1899) was used for the SN test and I-ELISA. The virus was propagated in TF104 cells (a cell line cloned from MA104 cell) in α-MEM containing 5% fetal bovine serum [19]. After 3 freezing and thawing cycles, the cell debris was removed by centrifugation at 3,000 g for 30 min. The viral supernatant was treated with polyethylene glycol (PEG, MW 8000; Sigma, USA) and precipitated at 4℃ overnight. The precipitate was diluted to 1/100 of the original volume with the GTNE buffer (200 mM glycine, 50 mM Tris, 100 mM NaCl, 1 mM EDTA, pH 7.5). The crude concentrates were then overlaid on a 10-50% (w/v) discontinuous sucrose gradient and centrifuged at 100,000 g for 3 h at 4℃ in an SW-41 rotor (Beckman, USA). The antigen was recovered from the interface between the two sucrose layers. After dialysis in phosphate buffered saline (PBS), the purified antigens were used for I-ELISA.
The sera from 132 sows in 6 farms were used to compare the I-ELISA results with both the HI and SN test, respectively. For the seroprevalence survey, an additional 720 serum samples from Gyeonggi (n = 270), Jeonbuk (n = 180), Gyeongnam (n = 180), Jeju (n = 90) in Korea were also collected from finishing pigs from July to August 2004. The serum samples were stored at -20℃ until needed. The JEV antibody status of the sera was analyzed using a HI test according to the standard method [5], which was modified for microtiter plates. The HI titer is expressed by the reciprocal of the highest dilution of serum that resulted in complete hemagglutination inhibition. An HI titer equal to or greater than 10 was considered positive.
The SN test with the inactivated sera at 56℃ for 30 min was performed in a 96-well microplates using TF104 cells. Serial two-fold dilutions of the sera were mixed with an equal volume of a KV1899 virus suspension containing 200 TCID50/0.1 ml. After incubating the virus-serum mixture at 37℃ for 1 h, the TF104 cells were added to the mixture to a final cell count of 20,000 cells/well. The plates were incubated at 37℃, under 5% CO2 for 5 days. Each well was examined microscopically for any cytopathic effect (CPE) and the neutralization titers were expressed as the reciprocal of the final serum dilution that prevented CPE in 50% of the cultures. A titer >4 was considered positive for the JEV antibody.
A checkerboard titration of each antigen pool and serum was designed for the optimal antigen and serum dilutions. The antigen and serum was diluted from 5 to 0.004 µg/ml and 1 : 20 to 1 : 10,240, respectively. The purified whole virus antigen was coated on a 96 well microplates (Maxisorp; Nunc, Denmark) in a carbonate buffer (pH 9.6) at 4℃ overnight. The wells were blocked with 200 µl of a blocking buffer (5% skim milk in PBS; Marvel Beverages, UK) at 37℃ for 1 h, which was followed by washing three times with PBS-T (PBS pH 7.2 containing 0.05% Tween 20). Fifty microliters of the diluted serum samples were added in duplicate to the antigen coated wells and incubated at 37℃ for 1 h. The plates were then washed with 200 µl of PBS-T, and 50 µl of the anti-swine IgG HRP conjugate (KPL, USA) diluted 1 : 4,000 in PBS were added to each well. After incubation at 37℃ for 1 h, an identical volume of the substrate ABTS (2.2'-azino-di-3-ethyl-benthizolinsulfonat; KPL, USA) was added. The plates were incubated for 15 min, and the chromogenic reaction was quenched by adding 50 µl of a 1% sodium dodecyl sulfate (SDS) solution. The absorbance was measured at 405 nm using a Sunrise ELISA reader (Tecan, Switzerland). The noisy value of the background absorbance of the blank was subtracted from the signal value of all the test readings. The specificity and sensitivity were calculated using the following formula; sensitivity (%) = (number of positives in both the test/total number of positives in the reference test) ×100, specificity (%) = (number of negatives in both tests/total number of negatives in the reference test) ×100.
The optimal conditions of the antigen and serum dilution were determined by cross titration using the sera from132 sows. Five percent skim milk in PBS was selected as the blocking buffer in order to reduce the number of nonspecific reactions. PBS-T was used as the washing and serum dilution buffer. The antigens were diluted from 5 µg/ml to 0.004 µg/ml with the coating buffer in order to determine the optimal antigen concentration. As shown in Fig. 1, there were distinct differences of absorbance between the positive and negative sera. The optimal serum dilution was determined using several serum samples diluted from 1 : 20 to 1 : 10,240 with PBS-T. There were differences between the positive and negative sera (Fig. 2). According to the results, the optimal concentration and dilution were found to be 1 : 40 for the antigen (1.25 µg/ml final concentration) and 1 : 200 in 1% skim milk of PBS-T for the serum. Sera with an absorbance >0.25 were considered positive.
The absorbance obtained from 132 serum samples were compared with the titers of their HI and SN tests. Comparative analyses showed a significant correlation between I-ELISA and the conventional HI (r = 0.867) and SN test (r = 0.804) (Fig. 3 and 4). Ninety-nine out of 105 positive serum samples by the HI test tested positive in the I-ELISA test, and 22 out of 27 negative serum samples by I-ELISA tested negative by the HI test (Table 1). When compared with the traditional diagnostic assays, the sensitivity of I-ELISA was 94.3% with the HI test and 93.7% with the SN test. In addition, the specificity was 81.4% and 80% with the HI and SN tests, respectively. Overall, the I-ELISA results showed a higher correlation with the HI test than with SN test.
Seven hundred twenty serum samples from finishing pigs were measured by I-ELISA to determine the seroprevalence of the JEV. As shown in Fig. 5, the total seroprevalence was 21.7% (156/720). The seroprevalence was relatively higher in Gyeongnam (45%, 81/180) than the other regions (12.2%, 11/90 in Jeju, 12.6%, 34/270 in Gyeonggi, 16.7% 30/180 in Jeonbuk, respectively).
The HI test has routinely been used for the sero-diagnosis of the JEV. However, the process used in the HI test requires the use of red blood cells obtained from geese. In addition, the production and preparation of antigens is restricted in certain laboratories for biosecurity reasons. Although the standard test for the SN test against the JEV is the plaque reduction neutralization (PRNT) [15], this assay is also time-consuming and requires a high level of expertise. The advantages of ELISA, compared with the traditional HI and SN test, are that it is easy to examine a large number of samples within a limited time and there is no requirement for specialized equipment [7,8,9]. In this study, I-ELISA was evaluated using the purified JEV as an alternative to the HI and SN test. The analysis, which was based on the distribution of the absorbance in 132 sow serum samples, produced 0.25 as a feasible borderline to differentiate between positive and negative samples. The comparative analysis of I-ELISA showed a high correlation with the HI test (r = 0.867), which suggests that I-ELISA can be used for the detection of antibodies against the JEV. These results correlate with the results reported by Xinglin et al. [17]. Swine sera have been reported to produce relatively high nonspecific signals in ELISA [7]. In this study, the nonspecific reaction was reduced considerably, which is attributed to the use of the purified antigen. The seroprevalence of the JEV using the HI test was reported earlier from different countries in Asia [2,12,16]. The field application of the I-ELISA method showed that 21.7% of screened pigs were seropositive against the JEV in Korea in 2004. Gyeongnam province (45%) showed the highest seroprevalence. Since I-ELISA in this study showed considerable sensitivity and specificity when compared with the HI test, it can be used for the sero-monitoring of a large amount of samples in the field. However, an ELISA methodology using recombinant proteins such as premembrane and envelope (prME), envelope (E) and NS1 protein will be required.
Figures and Tables
Fig. 1
Titration of the JEV antigen by I-ELISA using different concentrations of the purified JEV. The numbers show the HI titers of the pig sera.

Fig. 2
Titration of the serum by I-ELISA. The antigen was diluted to 1 : 40 (1.25 µg/ml) in 1% skim milk. The numbers show the HI titers of the pig sera.

Fig. 3
Comparison of the I-ELISA and HI tests for the JEV antibodies with the positive (105) and negative (27) sow sera.
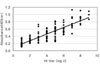
Fig. 4
Comparison of indirect ELISA and SN test for the JEV anribodies with 132 sow sera obtained from 6 farms.
Acknowledgments
The authors are grateful to Dr. Dong-Seob Tark for supplying the sera from the field and Dr. Seong-Hee Kim for her advice on this manuscript.
References
1. Burke DS, Monath TP. Fields Virology. 2001. 4th ed. Philadelphia: Lippincott;991–1024.
2. Chang KJ. Seasonal prevalence of anti-Japanese encephalitis virus antibody in pigs in different regions of Taiwan. J Microbiol Immunol Infect. 2002. 35:12–16.
3. Chen WR, Tesh RB, Rico-Hesse R. Genetic variation of Japanese encephalitis virus in nature. J Gen Virol. 1990. 71:2915–2922.

4. Chow VT, Yong RY, Ngoh BL, Chan YC. Automated type specific ELISA probe detection of amplified NS3 gene products of dengue viruses. J Clin Pathol. 1997. 50:346–349.

5. Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958. 7:561–573.

6. Kwon HJ, Lee CK, Kang BJ, Lim YM. Studies on Japanese encephalitis live vaccine. I. Isolation of Japanese encephalitis virus (Anyang strain) from a new-born piglet. Res Rep Off Rural Dev. 1974. 18:21–28.
7. Konishi E, Yamaoka M. Evaluation of enzyme-linked immunosorbent assay for quantitation of antibodies to Japanese encephalitis virus in swine sera. J Virol Methods. 1982. 5:247–253.

8. Konishi E, Yamaoka M. Rapid enzyme-linked immunosorbent assay of whole blood for detection of antibodies to Japanese encephalitis virus. J Virol Methods. 1983. 7:21–28.

9. Konishi E, Mason PW, Shope RE. Enzyme-linked immunosorbent assay using recombinant antigens for serodiagnosis of Japanese encephalitis. J Med Virol. 1996. 48:76–79.

10. Marx F, Gritsun TS, Grubeck-Loebenstein B, Gould EA. Diagnostic immunoassays for tick-borne encephalitis virus based on recombinant baculovirus protein expression. J Virol Methods. 2001. 91:75–84.

11. Paranjpe S, Banerjee K. Detection of Japanese encephalitis virus by reverse transcription/polymerase chain reaction. Acta Virol. 1998. 42:5–11.
12. See E, Tan HC, Wang D, Ooi EE, Lee MA. Presence of hemagglutination inhibition and neutralization antibodies to Japanese encephalitis virus in wild pigs on an offshore island in Singapore. Acta Trop. 2002. 81:233–236.

13. Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, Chin C, Lin TH, Huang JH. Antibody to the nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to differentiate infection from vaccination. Vaccine. 2001. 19:1753–1763.

14. Solomon T, Thao LT, Dung NM, Kneen R, Hung NT, Nisalak A, Vaughn DW, Farrar J, Hien TT, White NJ, Cardosa MJ. Rapid diagnosis of Japanese encephalitis by using an immunoglobulin M dot enzyme immunoassay. J Clin Microbiol. 1998. 36:2030–2034.

15. Ting SH, See E, Tan HC, Lee MA, Ooi EE. Development of a simplified assay for the detection of neutralizing antibodies to Japanese encephalitis virus. J Virol Methods. 2001. 93:43–47.

16. Ting SH, Tan HC, Wong WK, Ng ML, Chan SH, Ooi EE. Seroepidemiology of neutralizing antibodies to Japanese encephalitis virus in Singapore: continued transmission despite abolishment of pig farming? Acta Trop. 2004. 92:187–191.

17. Xinglin J, Huanchun C, Xiang W, Changming Q. Quantitative and qualitative study of enzyme-linked immunosorbent assay to detect IgG against Japanese encephalitis virus in swine sera. Vet Res Commun. 2005. 29:159–169.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download