Abstract
Blood-borne angiotensin-II (Ang-II) has profound effects in the brain. We tested the hypothesis that Ang-II-dependent hypertension involves differential Ang-II type I (AT1) receptors expression in the subfornical organ (SFO) and the rostral ventrolateral medulla (RVLM). Male Wistar rats were implanted with 14-day osmotic minipump filled with Ang-II (150 ng/kg/min) or saline. AT1 receptor mRNA levels were detected in the SFO and RVLM by reverse transcription-polymerase chain reaction (RT-PCR). Ang-II caused hypertension (134 ± 10 mmHg vs. 98 ± 9 mmHg, n = 9, p < 0.05). RT-PCR revealed that Ang-II infusion induced increased AT1 receptor mRNA levels in RVLM and decreased in SFO. Our data suggest that Ang-II-induced hypertension involves differential expression of brain AT1 receptors.
Blood-borne angiotensin-II (Ang-II) has profound effects on the central nervous system, including promotion of thirst, regulation of vasopressin secretion, and modulation of sympathetic outflow. In addition to blood-borne Ang-II, all components of the renin-angiotensin system are localized in the brain [11]. Within the brain, the renin-angiotensin system plays an important role in blood pressure regulation through its ability to modulate sympathetic nerve activity, especially in areas of the brain involved in the generation and modulation of the sympathetic discharge to cardiovascular system. Among those, two major areas are responsible for the sympathoexcitatory actions of Ang-II: the subfornical organ (SFO) and the rostral ventrolateral medulla (RVLM) [7,13].
The SFO is one of the circumventricular organs that lie outside the blood-brain-barrier and, thus, have access to circulating and intraventricular Ang-II. Moreover, angiotensinergic connections between the SFO and hypothalamic nuclei control drinking and modulate sympathetic nerve activity [6]. In addition to the SFO, the RVLM contains bulbospinal neurons that mediate a number of sympathetically mediated reflexes and contribute to some disease states caused by sympathoexcitation, such as hypertension [7]. Of note, a high density Ang-II type 1 (AT1) receptor is found in the RVLM [2] and microinjection of Ang-II into the RVLM produces an AT1 receptor-mediated enhancement in sympathetic activity resulting in increases in blood pressure [3,5]. Furthermore, the overexpression of AT1 receptors in the RVLM increases blood pressure [1], and the blockade of RVLM AT1 receptors reduces blood pressure in several forms of experimental hypertension [8,9,12].
Although various forms of hypertension are characterized by an elevation in Ang-II levels in the periphery, resulting in sympathetic overactivity, the signaling mechanism by which Ang-II reaches brain areas involved in blood pressure control to modulate the sympathetic nerve activity is largely unknown. In the present study we tested the hypothesis that increased circulating Ang-II levels activate AT1 receptors in the SFO, which in turn induce local production of Ang-II within the brain, increasing the Ang-II signaling in the rostral ventrolateral medulla by overexpression of AT1 receptors.
Adult male Wistar rats (250~280 g) were housed in a temperature-controlled room set to alternating 12-hour light-dark cycle with free access to standard rat chow (Labina; Purina, Brazil). All procedures were approved by the Federal University of Paraiba Animal Care and Use Committee, Brazil.
Rats were subcutaneously implanted with 14-day osmotic minipumps (Alzet; Durect Corporation, USA) filled with Ang-II (150 ng/kg/min) or saline. Therefore, animals were divided in two experimental groups: rats that were treated with saline (saline group, n = 25) and rats that were treated with Ang-II (Ang-II group, n = 28). After the drug infusion period, rats were anesthetized with ketamine and xylazine (75 and 10 mg/kg, intra-peritoneally, respectively) and fitted with a femoral arterial catheter for arterial pressure recordings, as described previously [3]. Briefly, blood pressure measurements were performed ≥ 24 h after catheter implantation. On the day of the experiment, the mean arterial pressure was recorded for 1 h in conscious rats using the software DataTrax 2 (LabTrax 2-24T; World Precision Instruments, USA).
In a subset of animals, rats were decapitated and brains were flash-frozen. Total RNA was extracted from fresh SFO and RVLM punches (five rats per biological sample from each group, run in triplicate) using TRIzol Reagent (Life Technologies, USA) according to the manufacturer's protocols. The cDNA species were synthesized using SuperScript II (Life Technologies, USA) from 1 µg of total RNA in a total volume of 20 µL with a random primer in accordance with the manufacturer's instructions. Real time PCR for AT1 receptors and β-actin (internal control) was performed as follows: the total volume was 25 µL, which was comprised of 1× SYBR PCR master mix, 300 nM of forward primer (5'-TAACATGAGCTCAGCCGCCAAA-3'), 300 nM of reverse primer (5'-TCGTGAGCCATTTAGTCCGATGCT-3') and 25 ng of cDNA. The PCR reaction was carried out in triplicate using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, USA) with the following thermal cycle parameters: 2 min at 50℃, 10 min at 95℃, 40 cycles of 15 sec each at 95℃ and 1 min at 60℃. The data were analyzed with Sequence Detection Software v1.4 (Applied Biosystems, USA). The ΔCt relative expression approach (relative expression = 2-ΔCt, where ΔCt = Ct(target gene)-Ct(control gene)) was employed to examine mRNA expression of AT1a receptors relative to β-actin.
Results are expressed as mean ± SE and were analyzed by the Student's t test. All statistical analyses were performed using GraphPad Prism (v. 5.0; GraphPad Software, USA). Statistical significance was defined as p < 0.05.
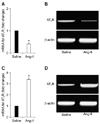
Rats treated with Ang-II presented a significant increase in blood pressure after 14 days of Ang-II infusion compared to rats infused with saline (134 ± 10 mmHg vs. 98 ± 9 mmHg, n = 13, p < 0.05). Changes in AT1 receptor mRNA levels in the SFO and in the RVLM (SFO being an important cardiovascular regulatory region that potentially influences Ang-II signaling events at the RVLM) caused by two weeks of peripheral infusion of Ang-II are presented in Fig. 1. AT1 receptor mRNA levels in the RVLM were significantly increased by approximately 240% at day 14 of the Ang-II infusion compared to the saline control group (fold change = 3.40 ± 0.50, n = 15). In contrast, AT1 receptor mRNA levels were significantly decreased in the SFO (fold change = 0.40 ± 0.20, n = 15) compared to saline control.
Here we report that chronic angiotensin-II infusion evoked an increase in AT1 receptor mRNA levels in the RVLM and decrease in AT1 receptor mRNA levels in the SFO as determined by real-time PCR. One important limitation of the present study is that we investigated the AT1 receptors expression only at the mRNA level. The confirmation of the AT1 receptors expression at the protein level by Western blot would be appropriate.
The important role of the SFO, a circunventricular organ involved in integrating signals from circulating angiotensin-II as well as local de novo production of this peptide, has been recognized [2,14]. In the present investigation, we also focused our attention on the RVLM because this brain region is the final relay point of the SFO-PVN-RVLM axis before transmission of signals generated by the brain renin-angiotensin system to the periphery [4]. Moreover, the RVLM controls sympathetic outflow and expresses renin and angiotensinogen locally [5,10].
Chronic Ang-II infusion also resulted in an increase in AT1 receptor mRNA levels in the RVLM as determined by quantitative real-time PCR, which may serve as evidence that enhanced Ang-II signaling within the RVLM results in hypertension. Increasing the expression of the AT1 receptors (up-regulation) in the RVLM would make the system more responsive to Ang-II released from angiotensinergic neurons, therefore increasing the firing rate of sympathetic neurons within the RVLM. Interestingly, AT1 receptor mRNA levels were decreased in the SFO of Ang-II infused rats. This phenomenon could be explained by the fact that increased circulating levels of Ang-II would reduce the expression of AT1 receptors (down-regulation) in that region, which is in intimate contact with the plasma Ang-II.
Here, using combined in vivo and molecular biology approaches, we report that the infusion of Ang-II induces differential AT1 receptor expression in two important areas of the brain involved in blood pressure and sympathetic activity regulation. The mechanism by which circulating Ang-II translates in sympathetic-mediated hypertension might involve changes in AT1 receptor expression in the RVLM and in the SFO.
Figures and Tables
Fig. 1
Real time-PCR analysis at 14 days of angiotensin-II (Ang-II) infusion reveals down-regulation of Ang-II type I receptors (AT1 R) in the subfornical organ (A and B) and up-regulation of AT1 R in the rostral ventrolateral medulla (C and D). *p < 0.05, when compared to saline group (n = 3, biological samples; 5 rat per sample).
Acknowledgments
The authors thank Mr. Antonio da Silva Santos for his technical assistance. This work was funded by the National Council for Scientific and Technological Development (CNPq; Grant # 451114/2009-4), Brazil.
References
1. Allen AM, Dosanjh JK, Erac M, Dassanayake S, Hannan RD, Thomas WG. Expression of constitutively active angiotensin receptors in the rostral ventrolateral medulla increases blood pressure. Hypertension. 2006. 47:1054–1061.

2. Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, Mendelsohn FAO. Angiotensin receptors in the nervous system. Brain Res Bull. 1998. 47:17–28.

3. Braga VA. Dietary salt enhances angiotensin-II-induced superoxide formation in the rostral ventrolateral medulla. Auton Neurosci. 2010. 155:14–18.

4. Dampney RAL, Fontes MAP, Hirooka Y, Horiuchi J, Potts PD, Tagawa T. Role of angiotensin II receptors in the regulation of vasomotor neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002. 29:467–472.

5. Dampney RAL, Tan PSP, Sheriff MJ, Fontes MAP, Horiuchi J. Cardiovascular effects of angiotensin II in the rostral ventrolateral medulla: the push-pull hypothesis. Curr Hypertens Rep. 2007. 9:222–227.

6. Eng R, Miselis RR. Polydipsia and abolition of angiotensin-induced drinking after transections of subfornical organ efferent projections in the rat. Brain Res. 1981. 225:200–206.

8. Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1999. 276:R1600–R1607.
9. Ito S, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support blood pressure in hypertensive rats. Hypertension. 2002. 40:552–559.

10. Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension. 2004. 43:1116–1119.

11. McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FAO, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003. 35:901–918.

12. Nunes FC, Ribeiro TP, França-Silva MS, Medeiros IA, Braga VA. Superoxide scavenging in the rostral ventrolateral medulla blunts the pressor response to peripheral chemoreflex activation. Brain Res. 2010. 1351:141–149.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download