Introduction
Bovine leukemia virus (BLV) is the viral agent of enzootic bovine leukemia (EBL) in cattle. With the exception of a few European countries, EBL is considered to have a worldwide distribution. Although the majority of infected cattle remain clinically asymptomatic, invisible losses in productivity have a significant economic impact on the dairy industry [4,24,26]. BLV is an oncogenic retrovirus of the Retroviridae family. Similar to other retroviruses, the envelope glycoprotein (Env) of BLV is the immunodominant protein in vivo [3,8,7,18]. The BLV env gene encodes a precursor protein that is processed into two subunits, gp51, the outer membrane subunit, and gp30, the transmembrane subunit, both of which are essential for viral infectivity [20, 28]. A variety of diagnostic tests have been developed for BLV, including PCR-based assays, agar gel immunodiffusions (AGIDs), virus neutralization assays, and enzyme-linked immunosorbent assays (ELISAs) [1,9,11,12,16,25]. The most widely used diagnostic methods for the serological detection of BLV-specific antibodies are AGID and ELISA, which are antigen-based assays that use either wild type BLV gp51 isolated from fetal lamb kidney (FLK) cells, or a recombinant BLV antigen that is expressed in insect cells [6,16,19,23]. Although the AGID test is less sensitive and specific than ELISA, AGID test has been widely used mainly for the routine diagnosis of serum samples because of the simplicity. However, the cell line FLK/BLV, used for antigen production of AGID, is known to be contaminated with bovine viral diarrhea virus (BVDV) [2,22]. Sometimes, cross-reactivity occurred between the BVDV antibodies induced by the vaccine and BVDV in the BLV antigen preparation.
The aim of this paper is therefore to describe the production of gp51 and partial gp30 by recombinant baculovirus in insect cells. The recombinant protein was used for detection of antibodies in sera of BLV-infected cattle as AGID antigen.
Materials and Methods
Cells and viruses
FLK cells chronically infected with BLV were cultured in Eagle's minimum essential medium containing 10% fetal bovine serum. To obtain BLV antigen for traditional AGID, FLK cell culture supernatant was collected and concentrated according to the protocol of Miller and Van Der Maaten [16].
For the cloning and expression of recombinant BLV gp51/gp30T-, Sf-9 and Hi-five cells were cultured in SF 900 II serum-free medium (Invitrogen, USA).
Cloning and expression of BLV gp51/gp30T-
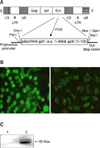
Recombinant BLV gp51/gp30T- (Korean isolate, Gene bank accession AY995174), which lacked the transmembrane region of gp30 and retained the signal peptide (amino acids 1-409) was expressed. Genomic DNA was extracted from the blood of dairy cows that were naturally infected with BLV using a genomic DNA kit (Promega, USA). For the amplification of BLV gp51/gp30T- sequences, the following oligonucleotide primers were used: 5'-CTC GAG ATG CCC AAA GAA CGA CGG TC -3', which contained a restriction enzyme site for Xho I at the 5' end, and 5'-GCT GGA GAT CAC CGA GGC GGA -3'. The amplified DNA fragment was inserted into pGEM T-easy (Promega, USA) for sequencing. The cloned DNA fragment was then excised and ligated into the transfer vector pBacPAK8 (Clontech, USA), as shown in Fig. 1A. A translational stop codon was inserted downstream of BLV gp51/gp30T- at the Pac I restriction site (5' TAATTA 3') of pBacPAK8 BlvGP. Recombinant viruses were purified by plaque assay and screened for the expression of BLV gp51/gp30T- by immunofluorescence assay (IFA), BLV-ELISA (indirect-ELISA), and western blot using BLV-positive bovine serum and monoclonal antibody as previously described [13-15].
Detection of recombinant BLV gp51/gp30T-
Indirect (I)-ELISA was performed as previously described [15]. Briefly, 100 µL of the supernatant from Hi-five cells infected with recombinant baculovirus and non-infected control cells were diluted 1 : 10 (2 µg/mL) in 0.05 M carbonate-bicarbonate buffer (pH 9.6). And the 96-well microplates (Maxisorp; Nunc, USA) were coated with the diluted supernatant at 4℃ overnight. Plates were blocked with 10% skimmed milk (Marvel, UK) in phosphate buffered saline (PBS) at room temperature for 1 h. Subsequently, plates were washed 3 times with PBS containing 0.05% tween 20 (PBST) and incubated with a 1 : 25 dilution of test sera, including the reference positive serum (NVSL, USA) in 10% skim milk in PBST for 1 h at 37℃. The plates were then washed 3 times with PBST. 100 µL of horseradish peroxidase labeled anti-bovine IgG (Pierce, USA) or each subclass (Serotec, UK), diluted 1 : 3,000 in PBST containing 10% skimmed milk, were then added to each well and incubated for 1 h at 37℃.
The plates were washed 3 times with PBST and developed with commercially available 3, 3', 5, 5'-tetramethyl-benzidine (Kirkegaard & Perry Laboratories, USA). After 30 min, the reaction was stopped by adding 100 µL of 0.5 M H2SO4. The optical density (OD) of the solution was measured at 450 nm. The net OD of test well was calculated by the subtraction of OD from control well.
The recombinant BLV gp51/gp30T- was tested for its reactivity to an anti-gp51 MAb by western blotting. The anti-gp51 MAb were produced through the cell fusion method [29]. Briefly, the DNA fragment for BLV gp51/gp30T- was ligated with pSecTaq 2C DNA vector (Invitrogen, USA) and intramuscularly inoculated to Balb/c mice. Anti- BLV gp51/gp30T- MAbs were screened and selected by indirect ELISA. One hybridoma cell line was finally selected for Western blotting in this study.
Preparation of recombinant BLV gp51/gp30T- for AGID
For the production of recombinant BLV gp51/gp30T-, approximately 7.5 × 107 Hi-five cells were infected with the recombinant virus (AcBlvGP) at a multiplicity of infection of 0.1-1, and then cultured at 27℃ for 6 days. Cultures were subjected to centrifugation at 3,000 × g for 30 min to remove cells, and then the supernatant was subjected to another round of centrifugation at 100,000 × g for 2 h. The supernatant was concentrated in a cellulose membrane (M.W. 12,000; GibcoBRL, USA) with polyethylene glycol (M.W. 8,000; Serva, Germany) for 12~18 h at room temperature. A fraction (1/100) of the initial supernatant volume was evaluated by AGID using the indicated reference sera. The concentration of recombinant antigen was determined using a BCA protein assay kit (Pierce, USA), according to the manufacturer's instructions, and antigen was stored at -70℃ until use. AGIDs were conducted as previously described [5,16,17].
Identification of the BLV provirus
For the detection of proviral DNA of BLV, DNA samples were prepared both from peripheral blood mononuclear cells (PBMCs) and whole blood by using commercially available DNA extraction Kit (Promega, USA). The first and nested PCR for proviral DNA of BLV was conducted according to the protocols described previously [1,17].
Animals and sera
A dairy cow (Holstein) that was naturally infected with BLV in the field was obtained from a farm in Korea. The cow was fertilized naturally, and the calf was placed in conventional housing with the dam, and monitored for the presence of BLV-specific antibodies, as previously described [24,26,27]. Whole blood samples and sera were collected at 3-week intervals.
The reference bovine sera (strong BLV-positive serum and negative serum) were purchased from National Veterinary Services Laboratories (USA). Two hundred and ten bovine serum samples that were collected at four slaughterhouses throughout Korea from 2003 to 2004 were also analyzed. The collected field sera were stored at -20℃ until use.
Diagnostic AGID
AGIDs were conducted according to the standard procedure recommended by the Office International des Epizooties [17]. Briefly, gel diffusion plates consisting of 0.8% noble agar and 8.5% NaCl were allowed to stand at room temperature for 72 h before obtaining a reading. All test sera were initially screened by AGID using baculovirus-expressed BLV gp51/gp30T- and FLK-derived BLV antigen. Sera were also tested using a commercially available ELISA kit (IDEXX, USA).
Data analysis
Calculations to determine test sensitivity and specificity were carried out as previously described [5]. Sensitivity and specificity were calculated according to the following equations:
% sensitivity = positive by both methods / (positive by both methods + positive by the standard and negative by the method being compared with the standard) × 100
% specificity = negative by both methods / (negative by both methods + negative by the standard and negative by the method being compared with the standard) × 100
Results
Expression of recombinant BLV gp51/gp30T-
DNA fragment for BLV gp51/gp30T- of BLV was amplified by PCR and the PCR product was inserted into the pGEM T-easy vector for sequencing. The nucleotide sequence of the amplified BLV gp51/gp30T- gene was compared with the previously published sequence (Gene bank accession AF503581) [28]. The sequence of BLV gp51/gp30T- showed 95.5% nucleotide identity and 96.0% amino acid identity, respectively (data not presented here). The BLV gp51/gp30T- fragment was ligated with pBacPAK8 by using Xho I and Xba I restriction sites (Fig. 1A). After cotransfection with viral DNA and pBacPAK8 BLV gp51/gp30T- into Sf-9 cells, recombinant clones were screened for the expression of BLV gp51/gp30T- by IFA and indirect-ELISA using positive bovine serum as indicated in Figs. 1B and 2. However, when the expressed cells and supernatant were compared at the same time by I-ELISA with positive bovine serum, the OD from the culture media was 10 times higher than the cells, showing that the expressed BLV gp51/gp30T- was secreted from the expressed cells (Fig. 2A). In fact, a time course experiment indicated that the recombinant BLV gp51/gp30T- was secreted outside of the expressed cells in 24 h and retained up to 120 h post inoculation in the supernatant indicating that it would be possible to prepare the recombinant BLV gp51/gp30T- by simple purification methods as shown in Fig. 2B. In addition, it was possible to detect the expressed BLV gp51/gp30T-, with a molecular weight of 62 kDa, from the supernatant with anti-gp51 MAb by western blotting as expected (Fig. 1C).
BLV diagnosis using recombinant BLV gp51/gp30T-
The AGID diagnostic potential was examined using recombinant BLV gp51/gp30T- as the antigen. The new antigen was serially diluted in duplicate and determined minimum concentration (data not present here). The minimum concentration was able to detect 0.3 mg/mL of BLV gp51/gp30T- (Fig. 2). The AGID results using recombinant BLV gp51/gp30T- were reproducible and consistent, and there were no significant variations in repeated tests. In the diagnostic AGIDs of a calf born to a BLV-infected mother, recombinant BLV gp51/gp30T yielded positive results at an earlier time point than the traditional antigen. The BLV antigen was detected the calf's whole blood by PCR (Table 1).
Field analysis of BLV antibody detection by AGID
A field evaluation of 210 serum samples was performed to determine the diagnostic efficiency of AGID using either recombinant baculovirus-expressed BLV gp51/gp30T- or traditional FLK-derived antigen. The same samples were analyzed using a commercially available diagnostic ELISA (IDEXX, USA). AGID using the recombinant BLV gp51/gp30T- detected 31 positive samples from a total of 43 positive sera (Table 2), whereas using the FLK-derived antigen detected 29 positive samples. The negative number for the recombinant is 179 (12 plus 167) and the negative number for the FLK is 181 (14 plus 167) (Fig. 3).
The diagnostic efficiencies of traditional and recombinant antigen-based AGIDs were compared to a commercially available BLV-ELISA. AGID using recombinant BLV gp51/gp30T- and traditional antigen had a relative sensitivity of 69.8% and 67.4%, respectively, and a relative specificity of 93.3% and 92.3% (Table 2). Overall, recombinant BLV gp51/gp30T- was more sensitive than the traditional FLK antigen in detecting BLV-positive sera. These results suggested that recombinant BLV antigen would be an effective tool for the sero-diagnosis of BLV.
Discussion
BLV gp51 and the BLV viral capsid protein (core protein, p24) are the immunodominant viral proteins in vivo. For this reason, the expression and purification of BLV gp51 for use as an antigenic reagent represents a promising approach to the development of diagnostic assays, as well as vaccines [6,21]. BLV env encodes a single precursor protein cleaved into two subunits; the outer membrane protein, gp51, and the transmembrane subunit, gp30. BLV gp51 is the major envelope glycoprotein, whereas gp30 contains an N-terminal fusion domain that participates in syncytium formation along with gp51, as well as a transmembrane domain [8,10,20,28]. In the current study, we have described the expression of recombinant gp51/gp30T- using a baculovirus expression system, in which the C-terminal transmembrane domain of p30 is lacking, and the purification of BLV gp51/gp30T- for use as a diagnostic antigen.
In contrast to BLV gp51, the BLV Env precursor protein is not secreted when it is expressed in insect cells [6,21]. In the current study, to eliminate the risk of antigen being retained in cells, we generated an env expression construct that encoded gp51 and the N-terminal region of gp30 lacking the C-terminal cytoplasmic domain and we demonstrated that BLV gp51/gp30T- was secreted from insect cells.
The FLK cell line is still the primary cell line used for BLV antigen production for commercial diagnostic tests. As this cell line is known to be contaminated with BVDV, the contamination may result in additional diagnostic problems concerning the specificity of the reactions [2,22].
Compared to the traditional production of BLV antigen derived from infected FLK cells, the current method is similar to previously described methods in that it is both simple and rapid [5]. Crude BLV gp51/gp30T- preparations were generated within a day from supernatants of spin-cultured insect cells, and provided sufficient antigen for more than 2,000 samples in a volume of 500 ml. There was no risk of adventurous viral contamination, as in the case with BVDV, and a low rate of variability from batch to batch might be achievable because of the simplicity in preparing AGID antigen. Furthermore, when we analyzed serum from a calf born to an infected mother, AGID with recombinant BLV gp51/gp30T- yielded positive results at an earlier time point than traditional AGID using FLK-BLV antigen. Recombinant BLV antigen proved to be more sensitive than the FLK/BLV antigen (by 2.4%). Overall, there was an increase in both in sensitivity and specificity in field tests of sera using recombinant BLV antigen, providing additional support for the use of recombinant BLV gp51/gp30T- as an alternative diagnostic antigen.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download