Abstract
The median time of brain metastasis from the diagnosis of breast cancer is approximately 3 years. In this case report, a 69-year-old woman demonstrated cerebellar ataxia. Brain magnetic resonance imaging revealed enhanced lesions in bilateral cerebellar hemispheres. She had undergone surgery, radiation, and chemotherapy for uterine and breast cancer 24 years prior and 16 years prior, respectively. Although she had not received any anticancer treatment for 10 years, no recurrences were identified using whole body scans. A partial tumor resection was performed and the histological diagnosis was an adenocarcinoma from breast cancer. As no extracranial lesions were found, gamma-knife irradiation was performed, without additional systemic chemotherapy. One month posttreatment, the tumors dramatically reduced in size and the patient completely recovered from cerebellar ataxia. Systemic chemotherapy is not always required for brain metastasis from breast cancer with a long interval period, as long as no evidence of extracranial recurrence is detected.
Brain metastasis commonly occurs in breast cancer and has been identified in about 20% to 24% of breast cancer patients [12]. The incidence of brain metastasis from breast cancer is increasing because patients with primary breast cancer are surviving longer [2]. Median time to brain metastasis is about 3 years with the longest reported interval of 97 months [123]. We report an extremely rare case of brain metastasis identified 16 years (193 months) after the diagnosis of breast cancer.
A 69-year-old woman visited a neurologist 1 month after the onset of dizziness. A neurological examination of the patient determined that she had cerebellar symptoms including mild dysarthria, poor finger-to-nose movement in the right hand, ataxic gait with wide-base steps while walking, and difficulty standing up from a chair without assistance. The patient had a history of multiple cancer diagnoses and had undergone surgery, chemotherapy, and radiation for uterine and bilateral breast cancer 24 years prior and 16 years prior, respectively. Annual follow-up examinations did not indicate cancer recurrence, even after discontinuation of anticancer treatments 10 years earlier.
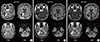
Brain magnetic resonance imaging (MRI) with gadolinium (gadopentetate dimeglumine) enhancement revealed lesions in the bilateral cerebellar hemispheres (Figure 1). Owing to the presence of multiple lesions, metastatic brain tumors were suspected. For the whole body examination, fluorodeoxyglucose-positron emission tomography (FDG-PET) was performed; however, no uptake of FDG was identified, even in the intracranial lesions (Figure 2A). Thallium-201 (201Tl) scintigraphy demonstrated uptake of Tl in the right cerebellar lesion in both early and delayed phases (Figure 2B). The retention index of the lesion was 1.73, suggesting a malignant character. For a histological diagnosis, a partial tumor removal was performed. The tumor was whitish in color, extremely hard, and invading into the normal surrounding cerebellar tissue (Figure 3). The histological diagnosis confirmed ductal adenocarcinoma (Figure 4A) that was estrogen receptor (ER), progesterone receptor (PR) and human epithelial growth factor receptor 2 (HER2) positive (triple-positive) (Figure 4B-4D), which was compatible with metastasis from breast cancer. Furthermore, the Ki-67 index was extremely high, 40% to 60% (Figure 4E). Gamma-knife radiation was performed on the residual cerebellar metastases because no other lesions were identified outside of the brain. One month after irradiation, a remarkable shrinkage of the tumors had occurred (Figure 5A, B). The patient recovered completely from the cerebellar symptoms and she was able to resume her normal activities. Until 5 months after the gamma-knife treatment was completed, neither recurrence of the cerebellar metastases nor other brain metastases have been identified (Figure 5C). The patient visits our clinic every 2 months and is walking smoothly without assistance.
Bone is the most common site of distant metastasis from breast cancer, followed by the lung, brain, and liver [4]. The cumulative frequency of breast cancer metastasis to the bone, lung, peritoneal, liver, and other sites does not differ among histological subtypes of breast cancer; however, the histological subtypes of breast cancer are significantly associated with different incidences of brain metastasis [5]. Additionally, the risks of brain metastasis from breast cancer include ER or PR expression negativity and high HER2 expression [67]. Indeed, HER2 was positive in this case, but both ER and PR were strongly positive as well, which are not considered to be high risk indicators for developing brain metastasis.
More than 50% of brain metastases from breast cancer cases demonstrate multiple brain lesions, and the cerebellum is a common metastatic site [2]. Furthermore, the occurrence of brain metastasis, without recurrence at any other site, is not extremely rare [3]. Considering all of these points, this appears to be a typical case of brain metastasis from breast cancer.
The prognosis for patients with brain metastasis from breast cancer is poor, and the median time to brain metastasis from the diagnosis of breast cancer is around 3 years [123]. Radiotherapy used to be a primary treatment for brain metastasis, but recently, chemotherapy and hormone therapy, such as tamoxifen [8], epirubicin and docetaxel [9], lapatinib and trastuzumab [10], or letrozole [11], were reported to contribute to longer survival periods, especially lapatinib as it is able to cross the blood brain barrier [10]. The difficulty in our case was to interpret the metastatic condition, considering the extremely long interval from the time of the breast cancer diagnosis to the occurrence of brain metastasis despite a high Ki-67 proliferative index. Two intracranial lesions were found, localized to only the cerebellum, and no other metastases or recurrences were identified. A continuation of systemic chemotherapy is frequently performed in patients with breast cancer who develop brain metastasis during adjuvant treatment [1112]. However, our case is different from terminal cancer cases because the original breast cancer had been well controlled, even after chemotherapy was discontinued 10 years earlier. Ogawa et al. [13] reported that in 45 patients with breast cancer and brain metastasis, improvements in neurological symptoms were obtained in 35 patients (77.8%) using palliative radiotherapy without systemic chemotherapy. Therefore, the therapeutic strategy focused on the brain metastasis, with the goal of diminishing the cerebellar lesions without the addition of any additional systemic therapy, to allow the patient to regain a good quality of life. Gamma-knife irradiation of the brain metastasis was selected as the treatment, which successfully decreased the size of the lesions and provided the patient with good quality of life outcomes.
Figures and Tables
Figure 1
Magnetic resonance imaging on admission. (A, B) Gadolinium (gadopentetate dimeglumine)-enhanced T1 weighted images revealed enhanced lesions in both cerebellar hemispheres (A, axial view; B, coronal view). The right side lesion was about 3 cm in longitudinal length and the left side was 8 mm. (C) The T2 weighted image showed the mass with iso- to high-signal with little brain edema around the tumor. (D) In the diffusion-weighted image the right tumor mass possessed an iso-signal but the left has a high signal. The forth ventricle was not compressed by the mass, suggesting the invading character of the tumor.
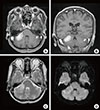
Figure 2
Radionuclide imaging for metastasis. (A) Fluorodeoxyglucose-positron emission tomography (FDG-PET). No evidence of metastasis was identified in a whole body scan. The uptake of FDG was much less in the right cerebellar hemisphere than in the left one. (B) Thallium 201 (201Tl)-scintigraphy. The uptake of Tl was only observed in the right cerebellar lesion in both early and delayed phases, and uptake was clearer in the delayed phase. Retention index (delayed/early) of the lesion was 1.73.

Figure 3
Surgical view. (A) The whitish extra-axial tumor (black arrow) can be seen attaching to the dura mater. (B) The tumor was extremely hard and firm without a clear border with normal brain tissue.
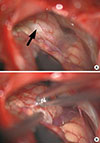
Figure 4
Histology. (A) Cellularity of the tumor is extremely high with a strong atypical appearance and mitosis that is compatible with adenocarcinomas (H&E stain, ×10 in the left, ×40 in the middle and the right). (B) The tumor presented estrogen receptor (IHC, ×20), (C) progesterone receptor (IHC, ×20), and (D) human epidermal growth factor receptor 2 (IHC, ×20) positive (triple-positive). (E) The Ki-67 proliferative index was extremely high, 40% to 60% (IHC, ×20 in the left, ×10 in the right).
H&E=hematoxylin and eosin; IHC=immunohistochemistry.
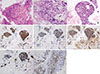
Figure 5
Follow-up magnetic resonance imaging (MRI) before and after surgery and gamma-knife radiation. (A) MRI before gamma-knife radiation. A small piece of tumor was resected and no sign of cerebral spinal fluid seeding was identified. (B) MRI 1 month after gamma-knife radiation. The size of the right cerebellar tumor decreased remarkably following treatment. The right irradiated lesion had a high signal in T2 weighted image (T2W) and a low signal in diffusion-weighted image (DWI), showing the necrotic change of the tumor. (C) MRI 5 months after gamma-knife radiation. The cerebellar metastatic lesions are still identified on MRI (Gd-T1W); however, the size of these lesions and edema around the tumor do not change at all.
Gd-T1W=gadolinium (gadopentetate dimeglumine)-enhanced T1 weighted image.
References
1. De Ieso PB, Schick U, Rosenfelder N, Mohammed K, Ross GM. Breast cancer brain metastases: a 12 year review of treatment outcomes. Breast. 2015; 24:426–433.
2. Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol. 2016; 127:407–414.

3. Brogi E, Murphy CG, Johnson ML, Conlin AK, Hsu M, Patil S, et al. Breast carcinoma with brain metastases: clinical analysis and immunoprofile on tissue microarrays. Ann Oncol. 2011; 22:2597–2603.

4. Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988; 81:1109–1112.
5. Park HS, Kim S, Kim K, Yoo H, Chae BJ, Bae JS, et al. Pattern of distant recurrence according to the molecular subtypes in Korean women with breast cancer. World J Surg Oncol. 2012; 10:4.

6. Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006; 17:935–944.

7. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015; 372:724–734.

8. Pors H, von Eyben FE, Sørensen OS, Larsen M. Longterm remission of multiple brain metastases with tamoxifen. J Neurooncol. 1991; 10:173–177.

9. Crivellari D, Pagani O, Veronesi A, Lombardi D, Nolè F, Thürlimann B, et al. High incidence of central nervous system involvement in patients with metastatic or locally advanced breast cancer treated with epirubicin and docetaxel. Ann Oncol. 2001; 12:353–356.

10. Larsen PB, Kümler I, Nielsen DL. A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Cancer Treat Rev. 2013; 39:720–727.

11. Nieder C, Oehlke O, Hintz M, Grosu AL. The challenge of durable brain control in patients with brain-only metastases from breast cancer. Springerplus. 2015; 4:585.

12. Madhup R, Kirti S, Bhatt ML, Srivastava PK, Srivastava M, Kumar S. Letrozole for brain and scalp metastases from breast cancer: a case report. Breast. 2006; 15:440–442.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download