Abstract
Purpose
The purpose of this study was to identify patients with high risk of distant metastasis (DM) after salvage treatment for postmastectomy locoregional recurrence (LRR).
Methods
We retrospectively reviewed 142 patients who received salvage radiotherapy with or without wide excision for isolated LRR after mastectomy between January 1999 and December 2012. Distant metastasis-free survival (DMFS) was estimated from the date of diagnosis of isolated LRR to the date of DM or last follow-up using the Kaplan-Meier method, and Cox regression analysis was performed to identify prognostic factors for DM.
Results
The median follow-up period was 54 months. The major failure pattern was DM (56%) and the 5-year DMFS was 43%. In multivariate analysis, initial N (iN) stage, recurrent N (rN) stage, and hormone receptor (HR) status were significant prognostic factors for DM (5-year DMFS: iN0 vs. iN1-3, 73% vs. 25%, p<0.001; rN0 vs. rN1-3, 61% vs. 29%, p<0.001; HR+ vs. HR-, 49% vs. 21%, p<0.001).
Postmastectomy locoregional recurrence (LRR) has been reported to be between 4% and 35% in breast cancer patients treated with mastectomy and no adjuvant radiotherapy depending on several risk factors [1234]. Although some patients with LRR showed long-term survival without any recurrence after thorough salvage treatments, most patients experienced second failures and showed poor prognosis [5678910]. Several retrospective studies, including our previous study, analyzed treatment results of postmastectomy LRR and reported that the major pattern of failure was distant metastasis (DM) [568910111213]. However, because of the small number and heterogeneity of the patients and the retrospective nature of these studies, prognostic factors for DM and the role of systemic therapy have not been well defined yet.
Recently, Aebi et al. [14] reported the results of the CALOR trial, which randomized patients with isolated LRR after mastectomy or lumpectomy either to chemotherapy or no chemotherapy. In that study, chemotherapy improved disease-free survival (DFS) and overall survival (OS) in the overall patient group and the estrogen receptor (ER) negative subgroup but not in the ER (+) subgroup. Although that was the only randomized trial which evaluated the role of chemotherapy, it seems to be insufficient to expand that results to all of patients with isolated LRR. The half of patients received lumpectomy at initial diagnosis and sites of isolated LRR were chest wall or in-breast in most patients (88%). Therefore, further studies are needed to evaluate prognostic factors for DM and the role of systemic therapy. In our present study, we investigated the prognostic factors for DM after salvage treatment for isolated LRR after mastectomy to estimate who might benefit from systemic therapy.
We retrospectively reviewed 142 patients who received salvage radiotherapy with or without prior wide excision for isolated LRR after mastectomy between January 1999 and December 2012 in our institution. Of these 142 patients, 71 were included in our previous report, which had a similar overall study design to that of our present study [10]. These 142 patients did not receive postmastectomy radiotherapy for the following reasons: (1) pT0-2N0-1 stage (n=115), (2) patient refusal (n=7), (3) immediate recurrence during adjuvant chemotherapy (n=1), (4) wound problem (n=1), and (5) unknown (n=18). When performing mastectomy, most patients (79%, 112 of 142) underwent axillary lymph node (LN) dissection. This study was approved by the Institutional Review Board of Asan Medical Center (approval number: 2015-0230).
Isolated LRR was defined as pathologically confirmed recurrences within the ipsilateral chest wall and/or regional lymphatics (axillary, supra-/infraclavicular, and internal mammary region) without simultaneous DM. The disease-free interval (DFI) was calculated from the date of mastectomy to the date of diagnosis of isolated LRR. The recurrent stage of isolated LRR as well as the pathologic stage of the initial tumor was assessed according to the American Joint Committee on Cancer (AJCC, seventh edition) TNM stage classification. The ER, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status in each case were examined by immunohistochemistry, and in situ hybridization was added when the HER2 status was equivocal by immunohistochemistry. If there was discordance between the initial mastectomy specimen and the LRR, we used the LRR result for analysis.
Radiotherapy was delivered to the involved field with or without an elective field using 4-15 MV X-ray and/or 6-16 MeV electron from a linear accelerator (Varian Medical Systems, Palo Alto, USA). The involved field was defined as the chest wall and/or regional lymphatic regions that contained LRR, and the uninvolved chest wall and/or regional lymphatic regions were considered elective fields. Total radiation doses were typically 4,500-5,080 cGy in 180-250 cGy fractions and various boost doses of 540-2,540 cGy were added to the tumor bed or gross tumor. Depending on the treatment volume, the two-field standard tangential technique, three-field technique, or reverse hockey stick technique was used. Hormone therapy and/or chemotherapy were performed depending on hormone receptor (HR) status and the physician's decision.
Distant metastasis-free survival (DMFS) and OS rates were estimated from the date of diagnosis of isolated LRR to the date of DM or last follow-up and to the date of death from any cause or last follow-up, respectively, by the Kaplan-Meier method. Univariate and multivariate analysis by the Cox proportional hazards model were performed to describe the association of independent variables with DMFS and OS. The independent variables included in the univariate analysis were age, initial pathologic T, N stage, DFI, recurrent T, N stage, HR status, HER2 status, wide excision, radiotherapy field, radiotherapy dose, and hormone therapy. Variables with p-values of <0.05 were included in the multivariate analysis and backward elimination Cox regression was used. All statistical tests were two-sided and performed at the 5% level of significance using SPSS version 18.0 (SPSS Inc., Chicago, USA).
Patient characteristics are summarized in Table 1. The median age was 43 years (range, 28-71 years) at initial diagnosis and 47 years (range, 30-74 years) at isolated LRR. The median DFI from the date of mastectomy to the date of diagnosis of LRR was 31.9 months (range, 1.4-159.1 months). The sites of recurrence were the chest wall in 63 patients (44%), axillary LN in 30 patients (21%), supra-/infraclavicular LN in 14 patients (10%), internal mammary LN in nine patients (6%), and multiple sites in 26 patients (18%). The T stage at recurrence was rT0, rT1, rT2, rT3, and rT4 in 44%, 37%, 7%, 1%, and 11%, respectively. The N stage at recurrence was rN0, rN1, rN2, and rN3 in 44%, 16%, 13%, and 26%, respectively.
Regarding the HR status, 112 patients (79%) were ER+ and/or PR+ and 29 patients (20%) were ER- and PR-. HER2 status was assessed in 132 patients (93%) and was positive in 44 patients (31%). Most patients (88%, 125 of 142) underwent wide excision before radiotherapy and 17 patients (12%) received radiotherapy alone (Table 2). Among the 125 patients who underwent wide excision, resection margin status was negative in 55 patients (44%), positive in 10 patients (8%), and unknown in 60 patients (48%). For regional recurrence, 67 of 79 patients received wide excision and the number of dissected LN was varied from 1 to 58 (≤6 in 37 patients and >6 in 30 patients). The median total radiation dose was 55 Gy (range, 38-70 Gy) and most patients (84%, 119 of 142) received elective field radiotherapy of the ipsilateral chest wall and regional lymphatic regions, as well as the tumor bed and gross tumor. Of the 142 patients, 91 (64%) received hormone therapy and 17 (12%) underwent chemotherapy. Forty patients (28%) did not receive any systemic therapy.
The median follow-up time was 53.8 months (range, 8.6-184.9 months). The major pattern of failure was DM with or without a second LRR in 79 patients (56%) and a second isolated LRR occurred in nine patients (6%). The 3- and 5-year OS rates were 82% and 66%, respectively (Figure 1A). The 3- and 5-year DMFS rates were 58% and 43%, respectively (Figure 1B). In univariate analysis, initial pathologic N stage (iN), DFI, recurrent N stage (rN), HR status, wide excision, and hormone therapy were significant prognostic factors for both OS and DMFS (Tables 3, 4). In multivariate analysis, iN stage (hazard ratio, 4.806; p<0.001), DFI (hazard ratio, 2.280; p=0.004), rN stage (hazard ratio, 3.125; p<0.001), and hormone therapy (hazard ratio, 2.415; p=0.003) were significant prognostic factors for OS (Table 3). Regarding DMFS, iN stage (hazard ratio, 4.683; p<0.001), rN stage (hazard ratio, 2.629; p<0.001), and HR status (hazard ratio, 3.062; p<0.001) were significant prognostic factors in multivariate analysis (Table 4). The 5-year DMFS rates were superior in patients with iN0 (73% vs. 25%, p<0.001), rN0 (61% vs. 29%, p<0.001), and HR (+) (49% vs. 21%, p<0.001) when compared with iN1-3, rN1-3, and HR- (Figure 2), respectively. In 112 HR+ patients, DMFS rates were significantly different depending on the iN stage and rN stage (5-year DMFS: iN0 vs. iN1-3, 77% vs. 31%, p<0.001; rN0 vs. rN1-3, 64% vs. 35%, p<0.001) (Figure 3).
In our present study, iN stage, rN stage, and HR status were significant prognostic factors for DMFS, and the 5-year DMFS rates were superior in patients with iN0 (73% vs. 25%, p<0.001), rN0 (61% vs. 29%, p<0.001), and HR+ (49% vs. 21%, p<0.001) than in patients with iN1-3, rN1-3, and HR-, respectively. The categorization of rN stage as rN0 versus rN1-3 is the same as the categorization of the site of recurrence as chest wall-only versus regional lymphatics with or without chest wall involvement. Several studies reported that chest wall-only recurrence is a good prognostic factor for DM [612]. Kamby et al. [6] reported that the median time to DM was longer in patients with chest wall-only recurrence than in patients with regional lymphatic recurrence (6.5 years vs. 3.7 years). The HR status in the recurrent specimen or initial mastectomy specimen was reported as a significant prognostic factor for DMFS in several studies [89]. Haffty et al. [8] examined PR status in chest wall recurrence specimens, and the 5-year DMFS rate was higher in patients with PR+ than in patients with PR- (84% vs. 38%). Regarding the iN stage, Kuo et al. [9] and Nielsen et al. [13] categorized the number of positive axillary LNs at the initial mastectomy as 0, 1-3, and ≥4 and showed prognostic value for DFS and DM, respectively. In several studies, a short DFI was reported as a poor prognostic factor for DMFS [81213] or DFS [9].
In addition to the effort to identify patients with high risk of DM, the role of systemic treatment for patients with isolated LRR after mastectomy has been evaluated in several studies. Regarding the role of hormone therapy, one randomized trial has been performed and showed superior DFS in tamoxifen group than in observation group [15]. However, the improvement in the DFS was mainly due to a decreased second LRR (p=0.011) and the incidence of DM and the OS rates were not significantly different between two groups. Regarding the role of chemotherapy, two prospective studies have been reported. In the nonrandomized prospective study of Haylock et al. [16], patients who received immediate chemotherapy at isolated LRR showed better DMFS (5-year DMFS, 75% vs. 61%) and OS (5-year OS, 82% vs. 74%) than patients who did not, but these differences were not statistically significant. However, the accrual periods were different between the chemotherapy group (from 1979 to 1983) and control group (from 1983 to 1989) and most patients were T1-2 (95%) N0 (85%) at initial mastectomy. These might act as a limitation to evaluated the role of chemotherapy for patients with LN involvement or >T2 stage at initial mastectomy. In 2014, results of the CALOR trial, which randomized patients with isolated LRR after mastectomy or lumpectomy either to chemotherapy or to no chemotherapy, were reported by Aebi et al. [14]. Chemotherapy significantly improved DFS and OS in the entire patients and ER- subgroup, but not in ER+ subgroup. Based on these results, the authors concluded that chemotherapy should be recommended in patients with isolated LRR, especially in patients with a HR- isolated LRR specimen. Although DFS was not improved by chemotherapy in HR+ patients and the role of chemotherapy seemed to be uncertain in those patients, it might be due to the favorable patient characteristics of that study in which only 12% of patients had regional recurrence and half of the patients received lumpectomy at initial diagnosis. Moreover, most of the patients (85%) had a DFI of 2 years or more. Because of these favorable characteristics, the number of events might be too small to detect a benefit of chemotherapy in HR+ patients. On the other hand, in our present study, a relatively large number of patients with regional recurrence (n=79, 56%) were included and all of the patients received mastectomy at initial diagnosis. We could see significantly different DMFS rates according to iN stage (5-year DMFS, iN0 vs. iN1-3, 77% vs. 31%) and rN stage (5-year DMFS, rN0 vs. rN1-3, 64% vs. 35%) in the subgroup of HR+ patients (n=112). Although the role of chemotherapy could not be evaluated in our present analysis because it was retrospective and only 12% of our patients underwent chemotherapy, it seems to be an important finding that patients who had LN involvement (at initial diagnosis or at recurrence) and/or were HR- had significantly worse DMFS. Further studies are needed to investigate the role of chemotherapy in these patients.
There were several limitations to the present study. First, because of its retrospective nature, treatment and patient characteristics were heterogeneous. Second, the number of patients who received chemotherapy was too small to evaluate the role of chemotherapy. Third, few patients received trastuzumab, one of the more recent treatment options, as salvage treatment for first isolated LRR. Nonetheless, the present study included a relatively large number of patients with both regional recurrence and chest wall recurrence.
In present study, patients with LN involvement and/or a HR- tumor seemed to experience more DM than patients with chest wall-only recurrence and a HR+ tumor. Further studies are needed to investigate the role of adjuvant chemotherapy in these patients.
Figures and Tables
Figure 1
Overall survival (OS) (A) and distant metastasis-free survival (DMFS) (B) rates in the entire patients.
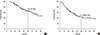
Figure 2
Distant metastasis-free survival (DMFS) rates depending on the (A) initial N (iN) stage, (B) recurrent N (rN) stage, and (C) hormone receptor status.

Figure 3
Distant metastasis-free survival (DMFS) rates depending on the (A) hormone receptor (HR) status and initial N (iN) stage, (B) hormone receptor status and recurrent N (rN) stage.
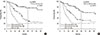
Table 1
Patient characteristics

AJCC=American Joint Committee on Cancer; LN=lymph node; ILRR=isolated locoregional recurrence; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Median (range); †Among 26 patients who had multiple site recurrences, 16 patients experienced both chest wall and LN recurrences and 10 patients had both axillary and supraclavicular LN recurrences.
Table 2
Treatment characteristics
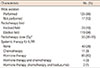
Table 3
Prognostic factors in overall survival

Table 4
Prognostic factors in distant metastasis-free survival

References
1. Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999; 17:1689–1700.

2. Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000; 18:2817–2827.

3. Wallgren A, Bonetti M, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Holmberg SB, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003; 21:1205–1213.

4. Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004; 22:4247–4254.

5. Magno L, Bignardi M, Micheletti E, Bardelli D, Plebani F. Analysis of prognostic factors in patients with isolated chest wall recurrence of breast cancer. Cancer. 1987; 60:240–244.

6. Kamby C, Sengeløv L. Pattern of dissemination and survival following isolated locoregional recurrence of breast cancer: a prospective study with more than 10 years of follow up. Breast Cancer Res Treat. 1997; 45:181–192.

7. Willner J, Kiricuta IC, Kölbl O, Flentje M. Long-term survival following postmastectomy locoregional recurrence of breast cancer. Breast. 1999; 8:200–204.

8. Haffty BG, Hauser A, Choi DH, Parisot N, Rimm D, King B, et al. Molecular markers for prognosis after isolated postmastectomy chest wall recurrence. Cancer. 2004; 100:252–263.

9. Kuo SH, Huang CS, Kuo WH, Cheng AL, Chang KJ, Chia-Hsien Cheng J. Comprehensive locoregional treatment and systemic therapy for postmastectomy isolated locoregional recurrence. Int J Radiat Oncol Biol Phys. 2008; 72:1456–1464.

10. Jeong Y, Kim SS, Gong G, Lee HJ, Ahn SH, Son BH, et al. Treatment results of breast cancer patients with locoregional recurrence after mastectomy. Radiat Oncol J. 2013; 31:138–146.

11. Deutsch M. Radiotherapy for postmastectomy local-regional recurrent breast cancer. Am J Clin Oncol. 2000; 23:494–498.

12. Moran MS, Haffty BG. Local-regional breast cancer recurrence: prognostic groups based on patterns of failure. Breast J. 2002; 8:81–87.

13. Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Loco-regional recurrence after mastectomy in high-risk breast cancer: risk and prognosis. An analysis of patients from the DBCG 82 B&C randomization trials. Radiother Oncol. 2006; 79:147–155.

14. Aebi S, Gelber S, Anderson SJ, Láng I, Robidoux A, Martín M, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014; 15:156–163.

15. Waeber M, Castiglione-Gertsch M, Dietrich D, Thürlimann B, Goldhirsch A, Brunner KW, et al. Adjuvant therapy after excision and radiation of isolated postmastectomy locoregional breast cancer recurrence: definitive results of a phase III randomized trial (SAKK 23/82) comparing tamoxifen with observation. Ann Oncol. 2003; 14:1215–1221.

16. Haylock BJ, Coppin CM, Jackson J, Basco VE, Wilson KS. Locoregional first recurrence after mastectomy: prospective cohort studies with and without immediate chemotherapy. Int J Radiat Oncol Biol Phys. 2000; 46:355–362.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download