Abstract
Purpose
Breast cancer is the most common malignancy of women worldwide. Radiotherapy consists of a vital element in the treatment of breast cancer but relative side effects and different radioactive responses are limiting factors for a successful treatment. Doxorubicin has been used to treat cancers for over 30 years and is considered as the most effective drug in the treatment of breast cancer. There are also many chronic side effects that limit the amount of doxorubicin that can be administered. The combined radio-drug treatment, with low doses, can be an approach for reducing side effects from single modality treatments instead of suitable cure rates.
Methods
We have studied the effect of 1, 1.5, and 2 Gy doses of 9 MV X-rays along with 1 µM doxorubicin on inducing cell death, apoptosis and also p53 and PTEN gene expression in T47D and SKBR3 breast cancer cells.
Results
Doxorubicin treatment resulted in upregulation of radiation-induced levels of p53 and downregulation of PTEN at 1 and 1.5 Gy in T47D breast cancer cells, as well as downregulation of p53 mRNA level of expression and upregulation of PTEN mRNA level of expression in SKBR3 breast cancer cell line. In addition, doxorubicin in combination with radiation decreased the viability of breast cancer cell lines in the both cell lines.
Breast cancer is the second leading cause of cancer-related deaths in women [1]. Compilation of the mutation data revealed two prevalent gene mutation patterns among the breast cancer cell lines (SKBR3 and T47D). The first pattern involved frequent mutations among the cell lines in genes from the same tumor suppressor pathway. These included the p53 pathway in 90% of the cell lines (p53), the RB pathway in 64% (p16) and the PI3K pathway in 56% (PTEN) [2]. The tumor suppressor protein p53 that is known as 'molecular policeman' [3] has been identified as a key regulator of cell metabolism for its modulation of the balance between the glycolytic and mitochondrial respiratory pathways [4,5] and regulate the cell cycle, inhibit angiogenesis and DNA repair and apoptosis [6,7]. Under normal cellular condition the p53 signaling pathway is in standby mode and its activation occurs in response to cellular stresses and leads to an increase in the level of p53 protein [6]. Breast tumors expressing a high level of p53 are more frequently in estrogen receptor (ER) negative and are associated with a higher proliferation rate and poorer survival rates [8,9]. Estrogen and ER play important roles in genesis and malignant progression of breast cancer. The p53 has the ability to regulate ERα expression [10]. Increasing evidence indicates that p53 dysfunction is an important event in breast cancer [11,12]. It is possible that the interaction between p53 and ER, resulting in their reciprocal regulations, plays an important role in adjusting normal breast epithelial cell proliferation and the aberration of this control may lead to breast cancer onsets and progressions [13]. On the other hand, PTEN expression is regulated at the transcriptional level by a set of transcriptional factors, including the p53, and at the posttranscriptional level by protein localization, modification, and degradation. PTEN has the ability to autoregulate its own expression and such autoregulation occurs through stabilizing p53. PTEN increases p53 protein level directly by increasing the half-life of p53. The physical interaction between PTEN and p53 is required for the transregulation of p53 with the PTEN expression and the phosphatase activities of PTEN are not necessary for the effect. To further evaluate whether the interaction between PTEN and p53 is required for PTEN autoregulation, a p53 COOH-terminal deletion mutant has been generated. It has been reported that p53 maintains the transactivation on its target genes; however, it loses 90% of the ability to interact with PTEN. This indicates that the COOH terminus of p53 has an inhibitory effect on its function. However, coexpression of PTEN with p53 has less effects in facilitating its transactivation than with wild type p53 [14]. Chemotherapy is frequently used to relieve symptoms in advanced breast cancer patients and to reduce the risk of recurrences for patients with localized breast cancer. Doxorubicin (adriamycin) has been used to treat cancers for over 30 years and it is an effective therapy [15] and is also considered as the most effective drug in treatment of breast cancer [15,16]. Monotherapy with doxorubicin has a good response rate of 10% to 50% [1], and doxorubicin-containing combination therapies usually result in better survival rate [17,18]. The drug is also used to treat a wide variety of solid tumors and hematological malignancies [19]. Doxorubicin is a member of cytotoxic anthracyclin antibiotics, a group of antibiotics that is known to cause generations of intracellular superoxide and hydrogen peroxide, which can mediate mitochondrial damage and apoptosis in a p53-independent manner, also induces cytotoxicity in tumor cells by developing reactive oxygen. Doxorubicin is obtained from Streptomycespeucetius, and can also be commercially synthesized. Doxorubicin forms a stable complex with DNA and topoisomerase IIα resulting in inhabitation of the normal function of the enzyme [20]. The complex enzyme is unable to relegate DNA strand breaks and thus, the DNA damage would be increased. Therefore, the increased p53 protein expression level in some cells after doxorubicin exposure is a response to doxorubicin-induced DNA damage [6].
However, there are many chronic side effects that limit the amount of doxorubicin that can be executed; the most detrimental side effect is the cardiomyopathy that may lead to irreversible congestive heart failures [3]. Common side effects include: "radiation recall" (can bring back skin damage from previous radiation therapy), decreased blood cell counts, increased risks of infection and bleeding, loss of appetite, stomatitis, alopecia (hair loss), nausea and vomiting, mouth sores, birth defects, liver toxicity, and acute arrhythmia. Cardiac toxicity becomes relevant at high doses [4] and high resistant [21]. In fact, the cardiac toxicity of doxorubicin may be related to the intracellular generations of reactive oxygen metabolites by the drug and antioxidants might diminish this toxicity while not hindering cell kills of lymphoid malignant tissue [14]. Doxorubicin induces single and double strand breaks in DNA mediated by topoisomerase II [20]. The ubiquitous expression of topoisomerases contributes to the nonselective targeting of doxorubicin, and is a major reason for the toxicity [18]. The toxicity of the drug can be reduced if it is used in conjunction with other more tumor-specific treatment modalities in order to reduce the dosage. The aim of this study is, therefore, to investigate the interactions between doxorubicin and radiotherapy on two different breast cell lines, namely SKBR3 and T47D, with different status of p53, PTEN expression and also ER.
The human breast cancer cell lines, SKBR3 and T47D (purchased from National Cell Bank of Iran, Pasteur Institute of Iran, Tehran, Iran), were grown in RPMI-1640 media supplemented with 10% heat-inactivated (50℃, 30 minutes) fetal bovine serum, penicillin (100 U/mL), streptomycin (100 µg/mL), and amphotericin B (0.25 µg/mL) at 5% CO2 and 95% air in a humidified 37℃ incubator.
Stock solutions of 1 M doxorubicin (Sobhan Chemotherapeutics Co., Tehran, Iran) were dissolved in PBS and diluted in culture medium to a final concentration of 1 µM doxorubicin and the cells treated for 24 hours prior to irradiation and subsequently incubated in triplicates [18,19]. A 9 MV linear accelerator X-ray machine (Linac Neptune, Warsaw, Poland) was used to irradiate the cells with doses of 100, 150, 200 cGy.
T47D and SKBR3 cells (7×103 cells/well for the both cell lines) were incubated in 96-well plates each containing 200 µL of supplemented cell culture media for 24 hours at 37℃ and 5% CO2. The cells were divided in four groups in triplicates: blank, doxorubicin, irradiation, doxorubicin/irradiation treated. In drug treated groups, the cells treated with a final concentration of 1 µM of doxorubicin for 24 hours. The rate of cellular proliferation was measured at 24 hours. Briefly, 10 µL of 5 mg/mL MTT (3-(4, 5-dimetylthiazol-2-yl)-2, 5-diphenyltrazolium bromide) (Roche Diagnostics GmbH, Mannheim, Germany) was added to each well. The cells were incubated at 37℃ and 5% CO2 for 4 hours and then the media was discarded and 200 µL of dimethyl sulfoxide was added to each well to solubilize the colored Formazan product and then 25 µL Sorenson buffer was added to each well as solubilizer buffer. Finally, absorbance was read using an ELISA plate reader (BioTeck, Bad Friedrichshall, Germany) at 570 nm wavelength. All the data calculated were analyzed relatively to the untreated cells and then normalized.
The induced apoptosis and necrosis of T47D & SKBR3 cells (7×103 cells), treated with the 1 µM doxorubicin and irradiation alone and with the combination, were measured using the Cell Death Detection ELISAPLUS kit (Roche Diagnostics GmbH, Mannheim, Germany). The procedure was performed according to the manufacturer's protocol. Briefly, supernatants and lysate of the cells were prepared and incubated in the microtiter plates coated with an antihistone antibody. The colored reactions were analyzed using an ELISA plate reader (BioTeck) at 405 nm wavelength.
Total RNA was extracted using RNX-Plus reagent (Cinagen Co., Tehran, Iran). Briefly, the cells (1×106 cells) were treated with 1 mL of RNX solution and incubated at room temperature for 5 minutes. After adding 200 µL of chloroform, the cell suspensions were centrifuged at 12,000 RPM at 4℃ for 15 minutes. Then, the upper phase was transferred to a new tube containing equal volume of isopropanol. After high speed centrifugation at 4℃ for 15 minutes, the supernatant was discarded and the RNA-pellet was washed with 1 mL of 75% ethanol. Finally, the RNA-pellet, after drying, was dissolved in diethyl phosphorocyanidate treated water. Concentration of purified RNA was determined by optical density at 260 and 280 nm wavelengths.
Reverse transcriptase reaction was performed using RevertAid™ First Strand cDNA synthesis kit (MBI, Fermentas, Lithuania). Briefly, RNA was treated with DNase I (Invitrogen, Carlsbad, USA) before cDNA synthesis to avoid DNA contamination. cDNA was retrotranscripted in 20 µL reaction solution containing 5 µg total RNA, reaction buffer, RNase inhibitor (20 unit), dNTP mix (20 nM), random hexamer primer, oligo (dt)18 primer, and 200 unit M-MuLV reverse transcriptase. Reverse transcription (RT) procedure was performed at 42℃ for 60 minutes and terminated by heating at 70℃ for 5 minutes.
Specific primer sequences designed for p53 and PTEN and β-actin ribosomal RNA using free online Primer BLAST software (fast prim6) and gene confirmation was done with GeneDetect® oligonucleotide gene probes (Oligo 3) in triplicates as following: PTEN primers: 5'-CAGAGCCAAGCGGCGGCAGA-3' (forward primer) and 5'-AGAAGCTGCTGGTGGCGGGG-3' (reverse primer), p53 primers: 5'-TGGGCGTGAGCGCTTCGAGA-3' (forward primer) and 5'-GGTGGCTGGAGTGAGCCCTGC-3' (reverse primer), β-actin primers: 5'-TCCCTGGAGAAGAGCTACG-3' (forward primer) and 5'-GTAGTTTCGTGGATGCCACA-3'(reverse primer). β-Actin rRNA was used as an internal control and the relative gene expression was measured by the 2-ΔCtformula: Expression Target Gene/β-actin Gen=(1+E)-CtTarget Gene/(1+E)-Ctβ-actin Gen. All real-time polymerase chain reaction (PCR) reactions were executed in 72-well reaction tubes containing 2x SYBER GREEN PCR master mix reagent (ABI, Vernon, USA), 190 nM primer and 1 µg cDNA in 20 µL reaction volume. Quantitative RT-PCR was performed using Corbett Rotor-Gene 6000 thermal cycler (Corbett Life Science, Sydney, Australia). The thermal cycle conditions were included one cycle of 95℃ for 5 minutes followed by 42 cycles of 95℃ for 20 seconds (denaturation) and 60℃ for 20 seconds (annealing) and 72℃ for 20 seconds (extension). At the end of each test, accuracy of reaction was confirmed by melting curve analysis with Corbett Rotor-Gene 6000 software.
MTT assay data were processed as medians. Nonparametric Mann-Whitney test was used to compare the death in test and control groups of SKBR3 and T47D breast cancer cells. Also the Spearman's correlation was used to find statistically significant correlations. Expression of target genes was obtained through the Corbett 6000 and expressed as Ct (cycle threshold), ΔCt (the subtraction of target and housekeeping genes). Amplification efficiency of each cytokine was being evaluated concerning 18s RNA expression as internal control and analyzing ΔCt variations of the template DNA dilutions. Graphs were plotted with the Graphpad Prism 6 (GraphPad Software Inc., San Diego, USA). Normal distribution of data was determined using P-P plot and statistically analyzed using statistical SPSS software version 16 (SPSS Inc., Chicago, USA).
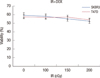
Figure 1A and B shows results of cell viability analysis of T47D and SKBR3 human breast cancer cells, incubated with 1 µM doxorubicin for 24 hours followed by radiation with the doses of 100, 150, and 200 cGy. Reduced cell viability was statistically significant for IR/DOXORUBICIN treated T47D cells at all radiation doses (p<0.05). The result for SKBR3 cell line were statistically significant at 100 and 200 cGy (p<0.05) but not at 150 cGy (p<0.12).
Results of the treatment-induced apoptosis and necrosis, obtained from ELISA analysis, were shown in Table 1 for SKBR3 and T47D breast cell lines.
Results of doxorubicin and radiation exposure on expression levels of p53 and PTEN mRNA in T47D and SKBR3 cell lines were analyzed using RT-PCR and the relative intensity of each band was measured and normalized with β-actin. The expression of p53 mRNA and PTEN mRNA is high in T47D and SKBR3 cell lines, respectively. The p53 mRNA expression slightly increased in T47D cell at different doses of radiation but decreased in SKBR3 cell line after doxorubicin and radiation treatment. In contrast, the PTEN mRNA expression slightly increased after radio-drugged treatments in SKBR3 cell at different doses of radiation but decreased in T47D cell line at the doses of 0 and 1.5 Gy compared with1 and 2 Gy of radiation (Table 2).
In T47D cell line as ER+, treatment with combination of doxorubicin and irradiation at the doses of 100 and 200 cGy increased expression level of the PTEN as well as irradiation alone. But in the doxorubicin treatment group alone and also with 150 cGy of radiation, the levels of PTEN mRNA expression have been lower than the controlled.
In SKBR3 cell lines, that are ER-, treatments caused high level of PTEN mRNA expression that asserts both of doxorubicin and radiation cause increasing of the gene expressions (Figure 2).
In the current study, effects on low dose of doxorubicin on cell viability, gene expressions, and radiation responses have been investigated in the malignant human breast cell lines (T47D, SKBR3). Doxorubicin, as an anthracycline antibiotic, is useful in a wide range of cancers and only a few cancer types are unresponsive to the drug. Cancers for which doxorubicin is used include: Hodgkin's and non-Hodgkin's lymphoma, breast cancer, ovarian cancer, testicular cancer, acute leukemia, soft tissue sarcoma, lung cancer, bladder cancer, gastric (stomach) cancer, thyroid cancer, hepatoma, Wilm's tumor, and neuroblastoma [19,22]. It shows effects on cancer cells via two different mechanisms. It acts as an intercalating agent and wedges between the DNA bases thus blocking DNA synthesis and transcription. The drug also inhibits the activity of an enzyme, topoisomerase type II [20]. This lead to breaks in the genomic DNA. Both of these mechanisms result in DNA disruption that ultimately can lead to the death of the cell. Common side effects include: "radiation recall" (can bring back skin damage from previous radiation therapy), decreased blood cell counts, increased risk of infection and bleeding, loss of appetite, stomatitis, alopecia (hair loss), nausea and vomiting, mouth sores, birth defects, liver toxicity, and acute arrhythmia. Cardiac toxicity becomes relevant at high doses. If present, the cardiomyopathy may lead to irreversible congestive heart failures [23]. In our study, a continuous presence of 1 µM doxorubicin for 24 hours had an effective killing influence on cells. Whereas the effects for combination of the doxorubicin and irradiation with the doses of 100, 150, and 200 cGy, with relation to the treatment dose per fraction in cancer radiotherapy, was higher than the doxorubicin. Breast cancer treatments have shown different cure ratios under similar conditions. There are undeniable evidences on different expression levels on a range of related oncogenes and suppressor genes, such as hdm2 and p53, in induction of a cancer. Therefore, treatment response differs in cancer for treatment modality. Compilation of the mutation data revealed two prevalent gene mutation patterns among the breast cancer cell lines (SKBR3 and T47D). The first pattern involved frequent mutations among the cell lines in genes from the same tumor suppressor pathway. These included the p53 pathway in 90% of the cell lines (p53), the RB pathway in 64% (p16) and the PI3K pathway in 56% (PTEN) [2]. Gene protein expression level studies have demonstrated that in breast cancer, there is overexpression of PTEN in SKBR3 breast cancer cells, as with the ER-negative cancer cells, containing the wild-type PTEN and normal expression of p53. Whereas, there is overexpression of p53 in T47D breast cancer cells, as ER-positive cancer cells, containing wild-type p53 and normal expression of PTEN. In addition to inducing genes that drive apoptosis, p53 can also activate the expression of genes that inhibits survival signaling (such as PTEN) or delay inhibitors of apoptosis (such as BIRC5) [24-26]. Also, the PTEN (phosphatase and tensin homologue), a dual specificity PIP3 phosphatase that antagonizes AKT signaling, is capable of blocking MDM2 nuclear translocations, thus preventing the negative effects of growth factors on p53 activity. PTEN may also be viewed as a tumor suppressor. The induction of PTEN has been shown to be essential for p53-mediated apoptosis in mouse cells, underscoring the importance of the AKT survival signaled in determining the final outcomes of the p53 response. The p53 increases PTEN and PTEN decreases AKT activity. Breast tumors with a high level of p53 expression are more frequently ER-negative and also with a higher proliferation rate and poorer survival rate. The ER-positives, due to lack of p53 function, often respond poorly to radiation and chemotherapy. In present study, combined radio-drug therapy at 100 cGy showed increased radiosensitivity in SKBR3 cell line, compared to T47D cell line, as a result of response to doxorubicin induced DNA damage in this cell line, and confirmed the results of Kaabinejadian et al. [6]. SKBR3 cell lines became more sensitive than T47D by increasing the doses of radiation. It seems that the high doses of radiation have significant effect on rapidly growing cells, SKBR3, compared to T47D. In ER-negative cell line (SKBR3) with an upregulation of p53, treatment with doxorubicin alone also caused the high expression of p53 mRNA.
The results on ER+ cell line (T47D), showed that the expression of p53 became higher after combined treatment in compare with control. In ER+ cell line, PTEN expression is unchanged but p53 expression decreased. Phosphates and tensin homolog (PTEN) (mutated in multiple and advanced cancers), located on 10q23.3, encodes a 403-residuedual specificity phosphatase that has both lipid and protein phosphatase activity [5,7,20,21]. Regulated movement of the tumor suppressors between nucleus and cytoplasm provides an efficient, simple, and rapid way for tumor suppressors to control cell growth [7]. PTEN participates in double-stranded-break repair, an interaction with CENP-C, which enhances centromere stability specifically, and overall genomic stability, and apoptosis [21].
The results showed an additive effect of lower doses of irradiation on T47D and support that this cell line is more sensitive compared with SKBR3 for low dose of radiation. But at 1.5 and 2 Gy of irradiation, SKBR3 cell line is more sensitive. Statistically significant correlations were observed between T47D and SKBR3 cell lines in doxorubicin and radiation treatment (p<0.05). Our study showed that 1.5 Gy radiation resulted to a limited increase of PTEN expression and decrease in p53 expression in T47D cells compared with 1 and 2 Gy radiation. A number of studies on the respond of cells to ionizing radiation have also shown different respond for similar conditions, and also for the genes expression [27-29]. Therefore, it seems to need some extended studies on the results.
This study demonstrate the ability of doxorubicin at low dose, 1 µM, as an effective treatment of breast cancer when used alone and also combined with ionizing radiation, also the treatment results of high dose irradiation can be achieved when combined with doxorubicin yet in low doses. Therefore, the combined treatment has benefit of decreasing treatment side effects with drug or irradiation alone from high therapeutic doses.
Figures and Tables
Figure 1
Effect of irrdiation alone and in combination with doxorubicin on viability of T47D (A) and SKBR3 (B) cells. The cells were treated with 1 µM doxorubicin and irradiated with doses of 100, 150, and 200 cGy.
IR=irradiation; DOX=doxorubicin.

Figure 2
Viability of T47D and SKBR3 cell lines following combined treatment with 1 µM doxorubicin and 100,150, 200 cGy irradiation.
IR=irradiation; DOX=doxorubicin.
ACKNOWLEDGEMENTS
We express our gratitude to the head and all staff members of the oncology department of Imam Reza Hospital of Tabriz University of Medical Sciences for their sincere cooperation. We wish to have a special thanks to Prof. Jafar Majidi Zolbanin, head of the immunology department, and Dr. Tohid Kazemi, head of the immunology lab, for providing a proper study condition.
Notes
References
1. Moulder S, Hortobagyi GN. Advances in the treatment of breast cancer. Clin Pharmacol Ther. 2008; 83:26–36.

2. Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M. Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res. 2007; 5:195–201.

3. Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994; 73:2013–2026.

4. Sinthupibulyakit C, Grimes KR, Domann FE, Xu Y, Fang F, Ittarat W, et al. p53 is an important factor for the radiosensitization effect of 2-deoxy-D-glucose. Int J Oncol. 2009; 35:609–615.

5. Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci. 2000; 899:349–362.

6. Kaabinejadian S, Fouladdel SH, Ramezani M, Azizi E. p53 expression in MCF7, T47D and MDA-MB 468 breast cancer cell lines treated with adriamycin using RT-PCR and immunocytochemistry. J Biol Sci. 2008; 8:380–385.

7. Schwarz SB, Schaffer PM, Kulka U, Ertl-Wagner B, Hell R, Schaffer M. The effect of radio-adaptive doses on HT29 and GM637 cells. Radiat Oncol. 2008; 3:12.

8. Feki A, Irminger-Finger I. Mutational spectrum of p53 mutations in primary breast and ovarian tumors. Crit Rev Oncol Hematol. 2004; 52:103–116.

9. Putti TC, El-Rehim DM, Rakha EA, Paish CE, Lee AH, Pinder SE, et al. Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol. 2005; 18:26–35.

10. Angeloni SV, Martin MB, Garcia-Morales P, Castro-Galache MD, Ferragut JA, Saceda M. Regulation of estrogen receptor-alpha expression by the tumor suppressor gene p53 in MCF-7 cells. J Endocrinol. 2004; 180:497–504.

11. Soussi T, Béroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001; 1:233–240.

12. Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005; 102:13550–13555.

13. Liu W, Konduri SD, Bansal S, Nayak BK, Rajasekaran SA, Karuppayil SM, et al. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J Biol Chem. 2006; 281:9837–9840.

14. Carter SK. C.R.O.S. conference on combined modalities chemotherapy/radiotherapy. Hilton Head Island, South Carolina, November 15-18, 1978. Cancer Chemother Pharmacol. 1979; 2:139–142.
15. Tan ML, Choong PF, Dass CR. Review: doxorubicin delivery systems based on chitosan for cancer therapy. J Pharm Pharmacol. 2009; 61:131–142.

16. O'Shaughnessy J. Liposomal anthracyclines for breast cancer: overview. Oncologist. 2003; 8:Suppl 2. 1–2.
17. Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007; 608:1–22.

18. Bergh J, Jönsson PE, Glimelius B, Nygren P. SBU-group. Swedish Council of Technology Assessment in Health Care. A systematic overview of chemotherapy effects in breast cancer. Acta Oncol. 2001; 40:253–281.
19. Hortobágyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997; 54:Suppl 4. 1–7.
20. Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984; 226:466–468.

21. Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol Med. 2005; 111:127–148.

22. Chabner BA, Ryan DP, Paz-Ares L, Garcia-Carbonero R, Calabresi P. Antineoplastic agents. In : Hardman JG, Lmbird LE, Gilman A, editors. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill;2001. p. 1389–1399.
23. Chanan-Khan A, Srinivasan S, Czuczman MS. Prevention and management of cardiotoxicity from antineoplastic therapy. J Support Oncol. 2004; 2:251–256.
25. Nakamura Y. Isolation of p53-target genes and their functional analysis. Cancer Sci. 2004; 95:7–11.

26. Lu X, Nannenga B, Donehower LA. PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev. 2005; 19:1162–1174.

27. Ju GZ, Shen B, Sun SL, Yan FQ, Fu SB. Effect of X-rays on expression of caspase-3 and p53 in EL-4 cells and its biological implications. Biomed Environ Sci. 2007; 20:456–459.
28. Sasaki A, Udaka Y, Tsunoda Y, Yamamoto G, Tsuji M, Oyamada H, et al. Analysis of p53 and miRNA expression after irradiation of glioblastoma cell lines. Anticancer Res. 2012; 32:4709–4713.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download