Abstract
Gastrointestinal metastases from invasive lobular breast cancer are uncommon with the stomach and small intestines being the most common metastatic sites. Peritoneal and rectal metastases are very rare and only rarely occur as the first manifestation of disease. We herein report the case of a 47-year-old woman who presented with abdominal carcinomatosis as a first sign of invasive lobular breast carcinoma (ILC). Identifying the most important immunohistochemical markers for ILC: gross cystic disease fluid protein 15, estrogen and progesterone receptors enabled a correct diagnosis. After a six year disease-free period, relapse occurred with severe obstruction due to rectal metastasis from lobular breast carcinoma. Since there was no widespread metastatic disease, surgery with concomitant hormonal therapy was performed.
Gastrointestinal metastases from invasive lobular breast carcinoma (ILC) are uncommon and usually occur years after the primary tumor. According to findings of large autopsy studies, the most frequent metastatic gastrointestinal (GI) sites are stomach, followed by small bowel and colon [1]. Interestingly, GI metastases were found to be the first manifestation of disease in clinically occult primary breast carcinoma. As ILC may present only as an asymmetric density rather than a dominant tumor mass [2], it could be missed on routine mammography evaluation. Histopathological and immunohistochemical analysis of biopsy specimen taken from GI metastatic sites are key in determining primary tumors [3]. Although GI metastases from ILC have been rare, the recognition of these entities is very important for making prompt diagnosis and providing appropriate treatment. Herein, we present a case of a patient with peritoneal carcinomatosis as a first sign of lobular breast carcinoma, with recurrence of disease six years later as rectal metastasis.
A 47-year-old premenopausal woman was admitted to our hospital due to abdominal distension, intermittent abdominal pain, and prolonged constipation. During a routine ultrasound (US) evaluation, ascites and complex cystic ovarian masses were detected. Colonoscopy revealed two extraluminal compressions, one in the region of proximal rectum and the other one in rectosigmoidal junction without any mucosal changes. Further abdominal computerized tomography (CT) examination showed peritoneal and omental implants, ascites, and bilateral ovarian cysts. Since the patient had complex ovarian cysts and ascites compatible with carcinomatosis, a diagnosis of ovarian cancer with peritoneal dissemination had been suspected.
The patient underwent total hysterectomy and bilateral salpingo-oophorectomy. Histological examination of ovarian and uterine tissue showed no malignant cells, while analysis of biopsy specimens taken from peritoneal and omentum implants revealed single-file strands of infiltrating small tumor cells, dispersed in the fibrous matrix with rare cells of the "signet-ring" type (Figure 1A). Further immunohistochemistry staining showed that 95% of cells were highly positive for cytokeratin 7 (CK7) and gross cystic disease fluid protein 15 (GCDFP-15). Further, estrogen (ER) and progesterone receptors (PR) were positive in 80% of tumor cells. These findings were consistent with a diagnosis of ILC with metastases to the peritoneum and omentum. Although mammography did not show the primary tumor, US examination revealed tumefaction in the left breast. A breast tumor biopsy showed grade 3 ILC (Figure 1B). The patient's human epidermal growth factor receptor 2 (HER2) status was negative: immunohistochemistry showed HER2: 2+, but chromogenic in situ hybridization analysis demonstrated that there was no gene amplification. Complete remission with regression of peritoneal and omental implants was achieved after chemotherapy which included fluorouracil, doxorubicine, and cyclophosphamide (FAC protocol). Levels of the cancer antigen 15-3 (CA 15-3), which was initially 66 U/mL (normal value <31), lowered to 19 U/mL. The patient continued on tamoxifen (20 mg/day) after cessation of chemotherapy with regular controls every six months.
After six years of disease-free period, the patient was admitted to our hospital for the second time because of prolonged constipation, early satiety, and weight loss. Rectosigmoidoscopy revealed thickening and rigidity of the rectal wall, with stenosis of the lumen 10 cm above the anal verge. Further examination with abdominal and pelvic magnetic resonance imaging (MRI) showed a stage 3 rectal tumor, with extension of tumor tissue through the muscle layer and obliteration of the interface between the muscle and perirectal fat (Figure 2). In order to determine the presence of distant metastases, the patient underwent a positron-emission tomography examination, which demonstrated a high uptake of fluorine18-fluoro-deoxy-glucose (18F-FDG) in the rectal wall, with an standardized uptake value (SUVmax) of 9.8 (Figure 3). Histopathological analysis of biopsy specimens taken during rectosigmoidoscopy showed diffuse infiltration of tumor cells along the rectal wall, some of them with the presentation of the "signet-ring cell" type (Figure 4A). Immunohistochemistry revealed that the tumor cells were reactive for CK7, GCDFP-15, CA 15-3, and ER (Figure 4B). On the basis of these findings, a diagnosis of rectal metastasis from lobular breast carcinoma was made. Clinical and mammography examination of the breasts excluded loco-regional relapse or second primary cancer in the contralateral breast. Since our patient had already developed stenosis and serious obstruction, rectal metastatic involvement was treated surgically with a colo-ano "pull-through" anastomosis, and subsequent operation after five months for closing a colostomy. In addition, daily treatment with aromatase inhibitor (anastrozole, 1 mg/day) was administered. After a one year follow-up period, the patient was asymptomatic and regular US and CT examinations have not shown a relapse of the disease. The clinical course of the disease has been depicted in Figure 5.
Gastrointestinal metastases from lobular breast carcinoma are infrequently recognized clinically, especially when occurring as a first manifestation of the disease. The predilection of ILC for metastasizing in the GI tract may be explained by its distinct histological and biological features. Berx et al. [4] found that the majority of ILC lack cohesiveness due to inactivation of E-cadherin, a cell-to-cell adhesion protein. Thus, metastatic spread could happen early in the disease course with barely detectable primary tumors as in our case. On the other hand, occurrence of GI metastases years after a primary tumor is probably related to ILC HER2 status. Overexpression of HER2 receptor in breast cancer has been associated with fast growth and a poorer prognosis [5]. ILC is typically a HER2 negative tumor which is consistent with its biological behavior, and patient surveillance.
Clinical manifestations of GI metastases from ILC have been quite diverse with nonspecific symptoms usually mimicking primary GI and pelvic malignancies. As described in previous reports, the most common initial diagnosis in patients with peritoneal metastases without known primary tumor has been ovarian cancer [6]. Similarly, in this case the patient presented with ascites and bilateral ovarian masses, and a diagnosis of ovarian cancer was made. Only after histopathological confirmation of metastatic breast cancer, the primary tumor was discovered. Although a median survival of a maximum 26 months for patients with peritoneal metastases from ILC has been previously reported [7], our patient experience six years of a disease free state. Given the fact that this was a highly endocrine responsive lobular breast cancer, it may be assumed that long disease free interval was mainly the result of a positive effect of endocrine therapy, ovariectomy, and tamoxifen [8]. Moreover, in previous studies peritoneal metastases were shown to be late with respect to manifestation of widespread disease, while in this report peritoneal carcinomatosis was the first sign of disease without other detectable metastases.
Rectal metastases from lobular breast carcinoma occur usually 5 to 7 years after primary tumors [9]. The most usual manifestation of rectal metastasis has been diffuse infiltration leading to thickening and rigidity of rectal wall [10], as in this report. Since clinical presentation of both rectal and peritoneal metastases is quite nonspecific, pathohistological and immunohistochemical analysis are the most important diagnostic tools in determination of diagnosis. Microscopically, metastases from ILC consist of spindle-shaped cells usually of the "signet-ring" cell type that show a single-file growth pattern with no dominant mass, as observed in our study [11]. The most important markers for ILC are GCDFP-15, ER and PR, which proved to be positive in biopsy specimens from peritoneal implants in our patient. Furthermore, tumor cells from the rectal wall were highly positive for CK7, GCDFP-15, and ER and negative for CK20 and PR markers. As stated previously, positivity for CK7 and negativity for CK20 suggest a metastasis, while CK7-/CK20+ profile characterizes a large bowel primary tumor [12]. To our knowledge, peritoneal carcinomatosis as a first manifestation of ILC with recurrence of disease in the form of rectal metastasis six years later in a single patient has not yet been described.
In conclusion, in any case of peritoneal carcinomatosis of unknown origin, ILC should be considered in the differential diagnosis. Furthermore, in patients with prolonged constipation and previous breast cancer history, awareness of rectal metastasis is necessary.
Figures and Tables
Figure 1
Histological features of peritoneal metastasis (A) and breast biopsy: invasive lobular breast carcinoma (B) (H&E stain, ×100).
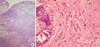
Figure 2
Turbo-spin-echo fat suppression T1 weighted (A) and turbo-spin-echo T2 weighted axial (B) and sagittal (C) MR image show a stage T3 rectal tumor. The tumor has intermediate signal intensity between the high signal intensity of the fat tissue and the low signal intensity of the muscular layer. Tumor signal intensity extends through the muscle layer into the perirectal fat, with obliteration of the interface between muscle and perirectal fat.
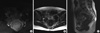
Figure 3
18F-FDG-PET/CT findings. (A) Axial computerized tomography (CT) image shows circumferential rectal wall thickening. (B) Axial CT attenuation-colorectal positron-emission tomography (PET) image and axial fused PET/CT image (C) revealed area of increased fluorine18-fluoro-deoxy-glucose (18F-FDG) uptake in rectal wall (standardized uptake value, SUVmax=9.8). (D) Increased uptake of 18F-FDG is also seen on maximum intensity projection reconstruction of CT attenuation-corrected PET image.

Figure 4
Histological and immunohistochemical features of rectal biopsy. (A) Diffuse carcinomatous infiltration of the rectal wall, mostly in the basal layer of mucosa and superficial submucosa. (B) Immunohistochemistry depicted strong immunoreactivity to gross cystic disease fluid protein 15 antigen (Immunohistochemical stain, ×100).
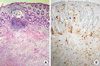
Figure 5
Clinical course of metastatic invasive lobular breast carcinoma. Signs and symptoms (☆) of the disease at the time of presentation and six years later. At first the patient underwent hysterectomy and salpingo-oophorectomy because of suspected ovarian cancer with peritoneal dissemination. After the diagnosis of metastatic invasive lobular breast cancer was made the patient received chemotherapy (FAC protocol) and tamoxifen remaining disease free for six years. The recurrence of disease was treated surgically with concomitant hormone therapy.

References
1. Winston CB, Hadar O, Teitcher JB, Caravelli JF, Sklarin NT, Panicek DM, et al. Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol. 2000. 175:795–800.
2. Sickles EA. The subtle and atypical mammographic features of invasive lobular carcinoma. Radiology. 1991. 178:25–26.

3. Sato T, Ohwada A, Miyaji A, Miyazaki R, Suzuki M, Matsumoto T. Immunohistochemistry for the differentiation of peritoneal disseminated carcinoma of unknown origin. Intern Med. 2004. 43:415–419.

4. Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996. 13:1919–1925.
5. Venanzi FM, Soverchia L, Felicetti P, Mennecozzi M, Concetti A. HER2/neu oncogene sequence revisited. J Natl Cancer Inst. 2002. 94:1808–1809.

6. Sheen-Chen SM, Liu YW, Sun CK, Lin SE, Eng HL, Huang WT, et al. Abdominal carcinomatosis attributed to metastatic breast carcinoma. Dig Dis Sci. 2008. 53:3043–3045.

7. Tuthill M, Pell R, Guiliani R, Lim A, Gudi M, Contractor KB, et al. Peritoneal disease in breast cancer: a specific entity with an extremely poor prognosis. Eur J Cancer. 2009. 45:2146–2149.

8. Katz A, Saad ED, Porter P, Pusztai L. Primary systemic chemotherapy of invasive lobular carcinoma of the breast. Lancet Oncol. 2007. 8:55–62.

9. McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005. 12:886–894.

11. Raju U, Ma CK, Shaw A. Signet ring variant of lobular carcinoma of the breast: a clinicopathologic and immunohistochemical study. Mod Pathol. 1993. 6:516–520.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download