Abstract
Purpose
To investigate the distribution of CD44+/CD24- cells in breast cancers in relation to tumor size before and after the administration of neoadjuvant chemotherapy.
Methods
CD44+/CD24- tumor cells obtained from breast cancer specimens were characterized in vivo and in vitro using tumor formation assays and mammosphere generation assays, respectively. The distribution of CD44+/CD24- tumor cells in 78 breast cancer specimens following administration of neoadjuvant chemotherapy was also evaluated using immunofluorescence assays, and this distribution was compared with the extent of tumor invasion predicted by Response Evaluation Criteria in Solid Tumours (RECIST).
Results
In 27/78 cases, complete remission (CR) was identified using RECIST. However, 18 of these CR cases were associated with a scattered distribution of tumor stem cells in the outline of the original tumor prior to neoadjuvant chemotherapy. After neoadjuvant chemotherapy, 24 cases involved cancer cells that were confined to the tumor outline, and 21 cases had tumor cells or tumor stem cells overlapping the tumor outline. In addition, there were 6 patients who were insensitive to chemotherapy, and in these cases, both cancer cells and stem cells were detected outside the contours of the tumor volume imaged prior to chemotherapy.
Preoperative chemotherapy has been used for the treatment of breast cancer since the 1970s, with Lyall et al. [1] being the first to apply neoadjuvant chemotherapy to the treatment of unresectable, locally advanced breast cancer. More recently, a study evaluated the use of curative resection following the administration of neoadjuvant chemotherapy for patients diagnosed with unresectable breast cancer [2]. However, in some cases, the efficacy of neoadjuvant chemotherapy was not sufficient to prevent tumor progression, and the delay associated with surgery was found to adversely affect a patient's condition [3].
In 1981, criteria were established that standardized the prediction of tumor invasion for therapeutic evaluations. These criteria have subsequently been modified by various cancer organizations including the World Health Organization (WHO), the National Cancer Institute, and the European Organization for Research and Treatment of Cancer [4]. Accordingly, a new set of tumor response criteria have been established, and are referred to as the Response Evaluation Criteria in Solid Tumours (RECIST) [5]. However, RECIST does not provide guidelines for the assessment of lesions classified as non-measurable lesions. Moreover, imaging studies have demonstrated that tumor images obtained can misrepresent the extent of present tumor [6]. Hence, the efficacy of the current RECIST in determining the extent of tumor invasion needs to act as re-evaluated. Correspondingly, the goal of this study was to investigate the capacity for cancer stem cells to be a readout for tumor invasion.
Previous studies have confirmed that tumor stem cells play an important role in tumor recurrence, metastasis, and chemotherapy resistance for various types of tumors, including breast cancers [7,8]. For example, in 2003, Al-Hajj et al. [7] indentified a small population of breast cancer stem cells within a breast tumor that were associated with tumor maintenance of the tumor. In addition, breast cancer stem cells have been shown to disseminate from a primary breast tumor and establish secondary tumors in distant organs such as the brain, liver, and lungs [7,8]. However, it remains unclear whether the biology or distribution of breast cancer stem cells is affected by neoadjuvant chemotherapy. It also remains unclear whether stem cells exist outside of a tumor according to the current criteria.
Currently, the distribution status of tumor stem cells before and after neoadjuvant chemotherapy remains controversial, as well as how this distribution relates to tumor size [9]. Therefore, the objective of this study was to investigate the population of CD44+/CD24- tumor cells present in primary breast cancers, and to predict the extent of tumor invasion based on this subset of cells using RECIST.
A total of 78 patients with histologically confirmed breast cancer underwent radical operations following neoadjuvant chemotherapy at The First Affiliated Hospital of Bethune Medical College, The First Hospital of the China Medical University and Liaoning Province Tumor Hospital between January 2007 and January 2011. The inclusion criteria were as follows: 1) curative operations were performed; 2) resected specimens were pathologically examined; 3) more than 15 lymph nodes were pathologically examined following an operation; and 4) a complete medical record was available.
The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Bethune Medical College, The First Hospital of the three Hospitals and Liaoning Province Tumor Hospital.
For flow cytometry analyses, fluorescence conjugated antibodies including: CD24-PE, CD44-FITC, CD2-FITC, CD3-APC, CD10-PE, CD16-FITC, CD18-APC, CD31-PE, and CD326-FITC (EpCAM) were used for FACS selection and were obtained from BD Pharmingen (San Diego, USA). Ultra-low adherent plates, sterile cell scrapers, MammoCult Basal Medium, MammoCult Proliferation Supplement, Hank's buffered salt solution (HBSS), hydrocortisone, and heparin were also purchased from Stem Cell Technologies (Vancouver, Canada).
Human mammary tissue (1×1×1 cm3 per specimen) was minced into 1×1×1 mm3 pieces with scalpels, washed three times with PBS, and transferred to tissue dissociation flasks. Tissue pieces were incubated with collagenase III at 37℃ and mixed using a 10-mL pipette every 15-20 minutes. After 3-4 hours, cells were filtered through a 45-µm nylon mesh and washed twice with PBS. Staining with trypan blue was used to count viable cells and to remove dead cells.
Flow cytometry was performed using a FACSVantage (FACSCALIBUR; BD Pharmingen). Cells were routinely sorted twice, then reanalyzed for purity, which typically was >95%. Briefly, cells positive for CD2, CD3, CD10, CD16, CD18, CD31, and CD326 were initially sorted out using flow cytometry. Dead cells were also eliminated according to staining detected with the viability dye, 7AAD. Subsequently, CD44+/CD24- tumor cells were selected, combined with RPMI-1640 medium and Matrigel (1:1 volume) and injected into the appropriate area of the mammary fat pad.
Complete MammoCult™ Medium (human) was supplemented with MammoCult™ Proliferation Supplements (human) and then added to MammoCult™ Basal Medium (Human). Cells were suspended in this prepared media before being plated on ultra-low attachment plates (Corning, USA) at a density of 2×104 viable cells/mL. The number of spheres that developed in each well was counted after 7 days.
Specimens (0.5×0.5×0.5 cm3) were resected at 1 cm intervals from the tumor diameter up to 3 cm outside the maximum diameter of the tumor. Briefly, breast cancer tissues and non-neoplastic breast tissues were prepared as paraffin blocks. Sections were cut (4 µm) and deparaffinized in xylene (2×, 5 minutes each), hydrated with 100% ethanol (2×, 3 minutes each), and incubated in 95% ethanol for 1 minute. Sections were then rinsed in distilled water and fixed in 3-4% paraformaldehyde in PBS (pH 7.4) for 15 minutes at RT. Samples were washed twice with ice-cold PBS and then incubated with PBS containing 0.25% Triton X-100 for 10 minutes. After three washes in PBS, samples were incubated with 1% bovine serum albumin (BSA) in PBS-Tween for 30 minutes to block non-specific binding. Sections were then incubated with rabbit anti-human CD44 and mouse anti-human CD24 antibodies in 1% BSA/PBS-Tween in a humidified chamber for 1 hour at RT, or overnight at 4℃. After three washes with PBS, sections were incubated with FITC-conjugated anti-rabbit and APC-conjugated anti-mouse antibodies in 1% BSA for 1 hour at RT in the dark. Sections were subsequently incubated with 0.1-1 µg/mL Hoechst or DAPI for 1 minute to stain for DNA. After adding a drop of mounting medium, sections were mounted with coverslips and sealed with nail polish.
CD44+/CD24-, CD44+/CD24+, and CD44- cell subsets were isolated from the tumor specimens collected using flow cytometry. CD44+/CD24- cells have previously been identified as breast cancer stem cells [7,10], and therefore, were combined with Matrigel to be injected into the mammary fat pad of NOD/SCID mice. After 20 days, tumors were detected in 3/5 mice (Figure 1). In contrast, tumors only formed in 1/5 mice which had been injected with 1×106 control cells (e.g., CD44+/CD24+ and CD44- cells) combined with Matrigel, compared to 5×103 CD44+/CD24- cells combined with Matrigel. Furthermore, CD44+/CD24- cells were observed to form mammospheres one week after being cultured in serum-free medium, while control cells did not (Figure 2).
When the distribution of CD44+/CD24- tumor cells determined from clinical examinations and radiological evaluations performed prior to the administration of neoadjuvant chemotherapy were compared with immunofluorescence studies performed on tissue sections obtained from surgical resection of tumors following neoadjuvant chemotherapy, differences in the localization of CD44+/CD24- tumor cells were observed. For example, prior to treatment with neoadjuvant chemotherapy, CD44+/CD24- tumor cells mainly localized to the edge of the breast tumors analyzed: However, following the administration of neoadjuvant chemotherapy, various distributions of CD44+/CD24- tumor cells were observed (Figure 3). For each of these patterns described below, a subgroup designation has been indicated in parentheses (i.e., Type I), the designations representing a proposed tumor invasion classification criteria that will be addressed in the Discussion (Figure 4).
Of the 78 cases, 27 were identified as being in complete remission (CR) according to RECIST. Moreover, of these 27 cases, 9 did not contain tumor cells (Type I), and 18 cases exhibited a scattered distribution of CD44+/CD24- tumor cells in the outline of the original tumor identified prior to neoadjuvant chemotherapy (Type II). Another 24 cases were associated with a distribution of cancer cells confined within the tumor outline identified following chemotherapy (Type III), while another 21 cases were associated with CD44+/CD24- tumor cells localized to the outline of the tumor following neoadjuvant chemotherapy. In the latter 21 cases, 9 involved tumor cells (Type IV) (Figure 5), 6 had tumor stem cells that also localized to the outline of the tumor following neoadjuvant chemotherapy (Type V), and 6 cases involved tumors that were insensitive to chemotherapy, resulting in the extension of cancer cells and stem cells outside the tumor outline established prior to chemotherapy (Type VI).
Currently, an increasing number of breast cancer patients are requesting that as much breast tissue as possible be maintained following a diagnosis of breast cancer [11]. Hence, the efficacy of the evaluation criteria used to determine the extent of tumor invasion has become very important in order to ensure the safety of the surgery, and to optimize the prevention of tumor recurrence. The decision to administer neoadjuvant chemotherapy for the treatment of breast cancer has been based on a clinical examination, a radiology evaluation including mammography, ultrasound, radionuclide, magnetic resonance imaging (MRI), and oxygen functional imaging, as well as pathology studies that include the detection and evaluation of molecular markers [12,13]. However, evaluation methods have been very limited for cases of breast cancer that involve a conservation approach for surgical resection since pathological studies cannot be performed for specimens outside conservatively designated surgical margins. Therefore, oncologists have had to depend on clinical examinations and radiology evaluation methods to evaluate the extent of existing tumor invasion. Therefore, the goal of this study was to evaluate the distribution of CD44+/CD24- tumor cells (which may represent an enriched population of breast cancer stem cells) for 78 cases of modified radical mastectomy that were performed following neoadjuvant chemotherapy according to the current criteria. To our surprise, we observed that some tumor cells, including CD44+/CD24- cancer stem cells, were scattered outside the tumor scope determined by currently accepted evaluation parameters for neoadjuvant chemotherapy.
Based on the results obtained from comparing tumor distributions prior to and following neoadjuvant chemotherapy, six patterns of distribution were identified. These patterns were labeled in the Results section, and represent a new tumor invasion classification system we propose based on the present study.
The proposed types include Type I and II, which relate to breast cancers considered to be in CR according to RECIST. Moreover, although Type II and imaging do not touch the body mass, there are foci there exhibited scattered tumor stem cells after chemotherapy. Types III- V were considered to represent tumors that exhibited a partial response (PR) to chemotherapy, or a stable disease (SD). In addition, Type III accurately reflected the extent of the tumor after chemotherapy. However, Type IV and Type V cases involved the presence of tumor cells outside the smaller tumor achieved following chemotherapy, especially in Type V cases. Furthermore, the distribution of stem cells in these cases also extended outside the currently accepted standard observable tumor invasion range. Overall, it is proposed that breast-conserving surgery is very dangerous for Type II and Type V breast cancer cases if tumor invasion is not carefully evaluated.
Usually, tumor resection surgeries include the collection of tissue specimens from the margin of the tumor resected, which are subsequently sent for pathological examinations to determine whether any residual tumor cells remain. However, accumulating evidence has indicated that these collected specimens do not accurately reflect the actual distribution of cancer cells. For example, neoadjuvant chemotherapy may reduce the overall tumor volume; however, residual tumor cells can still exist outside of the shrunken tumor, and just outside the surgical margin. Secondly, breast tumor cells have the potential to migrate to the duct, which was an aspect not fully considered in the present study. In contrast, the capacity for tumor stem cells to be insensitive to chemotherapy, radiation, or targeted therapy has been considered in the proposed Type II and Type V breast cancers, where local recurrence was associated with incomplete breast resections and residual tumor stem cells that remained.
In the past decade, the breast cancer stem cell theory has gradually been accepted by most oncologists [7,14]; however, research of breast cancer stem cells has come under unprecedented pressure to establish a stable breast cancer stem cell line in order to study the mechanisms involved. Research on breast cancer stem cells would be further improved by including considerations of disease control and treatment [15]. Although our study proposed clinical classifications for characteristics associated with tumor cell biology, the translation of these results into the clinic is limited by the relatively small number of cases and the short follow-up associated with each case evaluated. In addition, the determination of margins between breast cancer tissues and non-neoplastic breast tissues was made according to visual inspection during surgery due to technical limitations. Therefore, a larger number of breast cancer cases need to be analyzed, and need to include longer follow-up studies, in order to confirm the results of this study.
Figures and Tables
Figure 1
CD44+/CD24- cells (5×103) were combined with Matrigel and injected into the mammary fat pad of NOD/SCID mice. An example of a successfully established solid tumor is indicated with an arrow.
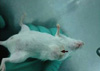
Figure 2
CD44+/CD24- cells were observed to form mammospheres one week after being cultured in serum-free medium (A: ×20), while control cells did not (B: ×20).

Figure 3
Various distributions of stem cells were observed following the administration of neoadjuvant chemotherapy. For example, few CD44+/CD24- tumor cells were observed in specimens from case no. 17 (A: ×10), while CD44+/CD24- tumor cells were observed to be distributed at the edge of the tumor in case no. 4 (B: ×10). Staining was performed with: mouse anti-human CD44 antibody and APC conjugated secondary (1); rabbit anti-human CD24 antibody with FITC conjugated secondary (2), and DAPI for the detection of nuclei (3). An overlay of panels (1), (2), and (3) is presented in (4).

Figure 4
Proposed tumor invasion classification criteria. Turquoise and magenta regions represent the tumor size identified before and after chemotherapy, respectively, while the red and blue circles represent the distribution of CD44+/CD24- and non-CD44+/CD24- tumor cells, respectively, associated with each type of tumor invasion.
Figure 5
For case no.12, tumor stem cells were observed to localize to the outline of the tumor following neoadjuvant chemotherapy (A: ×10). For case no. 25, cancer stem cells were detected in the vascular system of paracancerous tissues (B: ×10) and this was consistent with the results of immunofluorescence assays.

References
1. Lyall D, Schwartz M, Herter FP, Hudson PB, Wright JC, Findlay CW, et al. Treatment of cancer by the method of Revici. JAMA. 1965. 194:279–280.

2. Chong HY, Taib NA, Rampal S, Saad M, Bustam AZ, Yip CH. Treatment options for locally advanced breast cancer--experience in an Asian tertiary hospital. Asian Pac J Cancer Prev. 2010. 11:913–917.
3. Siponen ET, Vaalavirta L, Joensuu H, Vironen J, Heikkilä P, Leidenius MH. Ipsilateral breast recurrence after breast conserving surgery in patients with small (≤ 2 cm) breast cancer treated with modern adjuvant therapies. Eur J Surg Oncol. 2011. 37:25–31.

4. Smith BD, Buchholz TA, Kuerer HM. Intraoperative radiotherapy for early breast cancer. Lancet. 2010. 376:1141.

5. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. 45:228–247.

6. Linton KM, Taylor MB, Radford JA. Response evaluation in gastrointestinal stromal tumours treated with imatinib: misdiagnosis of disease progression on CT due to cystic change in liver metastases. Br J Radiol. 2006. 79:e40–e44.

7. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003. 100:3983–3988.

8. Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010. 140:62–73.

9. Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009. 15:4234–4241.

11. Yi M, Buchholz TA, Meric-Bernstam F, Bedrosian I, Hwang RF, Ross MI, et al. Classification of ipsilateral breast tumor recurrences after breast conservation therapy can predict patient prognosis and facilitate treatment planning. Ann Surg. 2011. 253:572–579.

12. Shin HJ, Kim HH, Ahn JH, Kim SB, Jung KH, Gong G, et al. Comparison of mammography, sonography, MRI and clinical examination in patients with locally advanced or inflammatory breast cancer who underwent neoadjuvant chemotherapy. Br J Radiol. 2011. 84:612–620.

13. Nakshatri H. Radiation resistance in breast cancer: are CD44+/CD24-/proteosome low/PKH26+ cells to blame? Breast Cancer Res. 2010. 12:105.
14. Oliveira LR, Jeffrey SS, Ribeiro-Silva A. Stem cells in human breast cancer. Histol Histopathol. 2010. 25:371–385.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download