Abstract
Primary breast lymphoma is a rare disease entity, particularly the T-cell type. There have been many case reports of primary breast lymphomas; however, these are mostly pathologic reports, with only a few reports in radiology literature. To the best of our knowledge, this is the first report on the radiologic features of primary T-cell type breast lymphoma, including mammography, ultrasonography, MR imaging, and 18 fluorodeoxyglucose positron emission tomography/computed tomography scan. The radiologic findings are rather unique for this T-cell lymphoma compared to B cell type.
Breast lymphoma is a rare disease entity worldwide, in either primary or secondary form, and makes up less than 0.5% of all breast malignancy.(1,2) Among all non-Hodgkin's lymphomas, only 0.7% show mammary tissue involvement.(2) Secondary forms of the disease are considered more common than primary ones,(3) with the primary forms representing 1.7% to 2.2% of all extranodal lymphomas,(4) probably attributing to less lymphoid tissue in the breast.(5)
There have been a number of pathologic case reports on primary breast lymphomas, however, only a few have been included in the radiology literature. Furthermore, the majority of reports are on the B-cell type, which constitutes the majority of primary breast lymphomas.(1,6) Published case reports on T-cell type primary breast lymphoma are very rare and published in pathology literature.
There have been no reports on the radiologic features of primary T-cell type breast lymphoma. In the present report, we describe a case of primary T-cell breast lymphoma, focusing on radiologic findings that include mammography, ultrasonography, MR imaging, and 18 fluorodeoxyglucose positron emission tomography/computed tomography (18 FDG-PET/CT) scan.
A 53-yr-old woman visited our hospital with a palpable mass at the upper outer quadrant of the right breast. The overlying skin was thickened and reddish in color. No skin retraction, nipple discharge, or palpable lymph nodes were observed in both axillae. Mammography (Figure 1) showed an ill-defined, irregular shaped hyperdense mass measuring 6.5×3.5 cm in size, accompanied by overlying skin thickening and ipsilateral lymphadenopathy. Ultrasonography showed no focal mass in either breast, but an ill-defined, hyperechoic lesion with tubular shaped branching hypoechogenecities was well-delineated in the palpable area (Figure 2). MR mammography (Figure 3) demonstrated an irregular 6.5×4.5 cm, ill-defined mass showing strong high signal intensity on T2 weighted images and slightly low signal intensities on T1 weighted images. Following intravenous administration of contrast materials, this lesion was well enhanced and showed a type III pattern time-signal intensity curve (early enhancement and delayed wash-out) on dynamic study. Several enlarged lymph nodes in the right axilla showed mild cortical thickening. F-18 FDG PET/CT (Figure 4) revealed a moderate hypermetabolic lesion at the upper outer quadrant of the right breast (maximum standard uptake value [SUV], 3.3-3.8) that was significantly lower in value compared with previously reported results. In addition, a focal hypermetabolic lesion measuring 2.3 maximum SUV was visible at the ipsilateral axilla.
The patient underwent ultrasound-guided core needle biopsy for the breast mass. Histopathologic examination (Figure 5) revealed diffuse infiltration of small and large lymphoid cells with admixed plasma cells. Immunohistochemical staining showed positive reaction for CD3 and CD43 in most lymphoid cells. Some CD20-posiive B cells are found, but negative for bcl-6, CD10 and cyclin D1. EBER-1 mRNA in situ hybridization was negative. T cell receptor gamma rearrangement study showed a monoclonal band.
Additional studies to evaluate systemic involvement were conducted. Chest X-ray and abdominal ultrasonography showed no abnormal findings.
Clinically, there were no generalized superficial lymphadenopathy, hepatosplenomegaly, or B symptoms. Bone marrow biopsy performed at the posterior iliac crest revealed hypocellular marrow with no involvement of malignant lymphoma. Total white blood cell count, lactate dehydrogenase, and B2 microglobulin levels were within normal limits.
The final diagnosis was primary breast lymphoma of the peripheral T-cell, not otherwise specified (NOS) type.
Primary breast lymphoma is rare, constituting only 1.7% to 2.2% of extranodal lymphomas and 0.38% to 0.7% of all non-Hodgkin's lymphomas. Criteria proposed by Wiseman and Liao(7) are now accepted as a standard method in differentiation of a primary breast lymphoma from a secondary form. These include 1) adequate pathological specimen, 2) anatomic association with breast tissue, and 3) lymphomatous infiltrate with no other lymphoma focus at the time of diagnosis, except for the presence of compromised ipsilateral axillary lymph node, and 4) absence of previous extramammary lymphoma. According to these criteria, most forms of breast lymphoma are secondary.(7) In our case, because the lesion located in the axillary tail where contains scanty breast tissue, breast tissue did not be included on the specimens. However, the main center of the lesion was a breast parenchyma rather than skin radiologically and there was a palpable breast mass without evidence of skin abnormality clinically. In addition, there was no other lymphoma focus at the time of diagnosis and absence of previous extramammary lymphoma. Therefore we could diagnose to primary breast lymphoma.
Lyou et al.(8) reviewed the mammographic and ultrasonographic images of 12 patients with primary B-cell type breast lymphoma. Most descriptions of the mammographic features of lesions include an oval shape (72.7%) with high density (90.9%). On ultrasonography, B-cell lymphomas were usually single (75%), circumscribed (50%), or microlobulated (37.5%), and oval in shape (50%). Yang et al.(9) described image findings of 32 breast lymphomas in 27 patients. Common ultrasonographic features included irregular, hypoechoic, and hypervascular tumors with indistinct margins or echogenic boundaries. PET/CT scans were available in 13 tumors of 10 patients; 12 of these tumors (92%) showed intense, diffuse hypermetabolic activity (mean maximum SUV of 10.6) with response to therapy on PET/CT scan after 8 weeks in all cases. In our case, ultrasonographic finding was an ill defined lesion with ductal changes and the SUV of the lesion on PET/CT scan was much lower than B-cell lymphoma. Although the reason is unclear so far, from the study by Kako et al.,(10) maximum SUV varied widely even among patients with the same histological subtype of T-cell lymphoma. Otero et al.(11) mentioned that the FDG avidity of peripheral T-cell lymphoma, NOS, lesions at PET/CT is high with the possible exception of cutaneous and bone marrow involvement. The maximum SUV of our current case is 3.3 and this is possibly due to the involvement of subcutaneous and skin layer. On the follow-up PET/CT scan after chemotherapy, the SUV was decreased from 3.3 to 1.0 at the breast lesion and disappeared at the lymph node in the ipsilateral axilla.
To the best of our knowledge, no reports have referenced radiologic findings showing the T-cell phenotype. Our present case demonstrated unique radiologic findings compared to the B-cell type of lymphoma. Mammogram in this case showed that the tumor was an ill-defined, irregular hyperdense mass with a diffuse hyperechoic area containing internal tubular branching hypoechogenecities without focal mass that mimic inflammation on ultrasonography. Other differences included skin thickening, possibly reflecting cutaneous extension of the disease and ipsilateral axillary lymphadenopathies. PET/CT showed a moderate hypermetabolic lesion (maximum SUV, 3.3-3.8) of significantly lower value compared with previously reported results.
In conclusion, primary breast lymphoma of the T-cell type is exceptionally rare. This is the first report demonstrating radiologic findings that differ somewhat from the radiologic characteristics of the B-cell type, particularly on ultrasonography and PET/CT scan. Further imaging investigation is needed for a better understanding of this rare entity.
Figures and Tables
Figure 1
Mammography shows an ill-defined, irregular shaped hyperdense mass (arrow) in the right upper outer quadrant, accompanied by slight thickening of the overlying skin and ipsilateral lymphadenopathy (arrowhead).

Figure 2
Ultrasonography shows an ill-defined hyperechoic lesion (arrow) with tubular shaped branching hypoechogenecities (arrowhead) without focal mass.
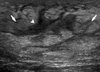
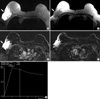
Figure 3
MRI demonstrates an ill-defined irregular mass showing very high signal intensity on T2 WI (A, arrow) and low signal intensity on T1 WI (B, arrow) in the upper outer quadrant of right breast. Early subtraction images (C, D) show a well-enhanced mass (C, arrow). An enhancing enlarged lymph node is visible in the right axilla (D, arrowhead), with well-delineated skin thickening. This shows a type III pattern time-signal intensity curve (early enhancement and delayed wash-out) on dynamic study (E).
References
1. Arber DA, Simpson JF, Weiss LM, Rappaport H. Non-Hodgkin's lymphoma involving the breast. Am J Surg Pathol. 1994. 18:288–295.

2. Uesato M, Miyazawa Y, Gunji Y, Ochiai T. Primary non-Hodgkin's lymphoma of the breast: report of a case with special reference to 380 cases in the Japanese literature. Breast Cancer. 2005. 12:154–158.

3. Petrek JA. Harris JR, editor. Lymphoma. Breast Diseases. 1991. 2nd ed. Philadelphia: Lippincott;806–807.
4. Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972. 29:252–260.

5. Fukutomi T, Makuuchi M, Itabashi M, Tobinai K, Nanasawa T, Yamamoto H, et al. A rare case of asynchronous bilateral B-cell lymphoma of the breast. Jpn J Clin Oncol. 1989. 19:391–396.
6. Jeon HJ, Akagi T, Hoshida Y, Hayashi K, Yoshino T, Tanaka T, et al. Primary non-Hodgkin malignant lymphoma of the breast. An immunohistochemical study of seven patients and literature review of 152 patients with breast lymphoma in Japan. Cancer. 1992. 70:2451–2459.

8. Lyou CY, Yang SK, Choe DH, Lee BH, Kim KH. Mammographic and sonographic findings of primary breast lymphoma. Clin Imaging. 2007. 31:234–238.

9. Yang WT, Lane DL, Le-Petross HT, Abruzzo LV, Macapinlac HA. Breast lymphoma: imaging findings of 32 tumors in 27 patients. Radiology. 2007. 245:692–702.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download