Abstract
Literature Review Summary
Electromagnetic field (EMF) is known to modify some relevant physiological parameters of cells cultured in vitro, such as proliferation, synthesis, secretion of growth factors and transcription. EMF induces bone formation in delayed, non union and spinal fusion models. A lso, the exposure of EMF has been shown to protect against the hazardous effect of smoking in the rabbit IVD.
Literature Review Summary
Electromagnetic field (EMF) is known to modify some relevant physiological parameters of cells cultured in vitro, such as proliferation, synthesis, secretion of growth factors and transcription. EMF induces bone formation in delayed, non union and spinal fusion models. A lso, the exposure of EMF has been shown to protect against the hazardous effect of smoking in the rabbit IVD.
Materials and Methods
Human IVD cells were Three-dimensionally cultured in alginate beads and exposed to a 650Ω, 1.8mil-litesla magnetic flux density, 60Hz sinusoidal wave of EMF. The cultures were divided into the control and EMF groups, with various exposure times. The cytotoxicity, and DNA and proteoglycan syntheses were measured by the MTT assay, and [3H]-thymidine and [35S]- sulfate incorporation, respectively. RT-PCRs were performed for aggrecan, and collagen types I and II mRNA expressions.
Results
There was no recognizable cytotoxicity in the EMF group, but cellular proliferation was stimulated (p<0.05). Newly synthesized proteoglycan, normalized by DNA synthesis, was decreased in the EMF group (p<0.05) as were the expressions of aggrecan (48hour exposure) and type II collagen (72 hours exposure) mRNA compared to the control group.
REFERENCES
1). Fontanesi G, Dal Monte A, Rinaldi E, et al. The effect of low frequency pulsing of an electromagnetic field for the treatment of congenital and acquired pseudoarthrosis. J Bioelectricity. 1984; 3:155–175.
2). Traina GC, Fontanesi G, Costa P, et al. Effects of electromagnetic stimulation on patients suffering from non-union. A retrospective study with a control group. J Bioelectricity. 1991; 10:101–117.
3). Brighton CT. Advanced clinical applications of electromagnetic field effects: bone and cartilage. Brighton CT, Pollack SR, editors. Electromagnetics in biology and medicine. San Francisco Press;San Francisco: p. 293–308. 1991.
4). Guizzardi S, Di Silvestre M, Govoni P, Scandroglio R. Pulsed electromagnetic field stimulation on posterior spinal fusions: a histological study in rats. J Spinal Disord. 1994; 7:36–40.
5). Goodman EM, Greenebaum B, Marron MT. Effects of electromagnetic fields on molecules and cells. International Rev Cytol 1. 1995; 58:279–338.

6). Hinsenkamp MG, Rooze MA. Morphological effect of electromagnetic stimulation on the skeleton of fetal or newborn mice. Acta Orthop Scand(suppl). 1982; 196:39–50.

7). Smith RL, Nagel DA. Effects of pulsing electromagnetic fields on bone growth and articular cartilage. Clin Orthop. 1983; 181:277–282.

8). Diniz P, Soejima K, Ito G. Nitric oxide mediates the e.ffects of pulsed electromagnetic field stimulation on the osteoblast proliferation and differentiation. Nitric Oxide. 2002; 7:18–23.

9). Chang K, Chang WHS. Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model: A prostaglandin E2-associated process. Bioelectromagnetics. 2003; 24:189–198.

10). Libo AR, Williams TJ, Strong DM, Wistar RJ. Time varying magnetic fields: Effect on the DNA synthesis, Sci -ence. 1984; 223:818–819.
11). Shomura K. Effects of pulsing electromagnetic field on the proliferation and calcification of osteoblast-like cell line, MC3T3-E1, J. Jpn. Orthod. Soc. 1997; 56:211–223.
Fig. 1.
Electromagnetic field (EMF) generator A; Power source B; EMF generator C; Culture plate in EMF generator. EMF was exposed to IVD cells with 650, 1.8 millitesla magnetic flux density, 60Hz sinusoidal wave. Cultures were divided into control and EMF group with various exposure times.
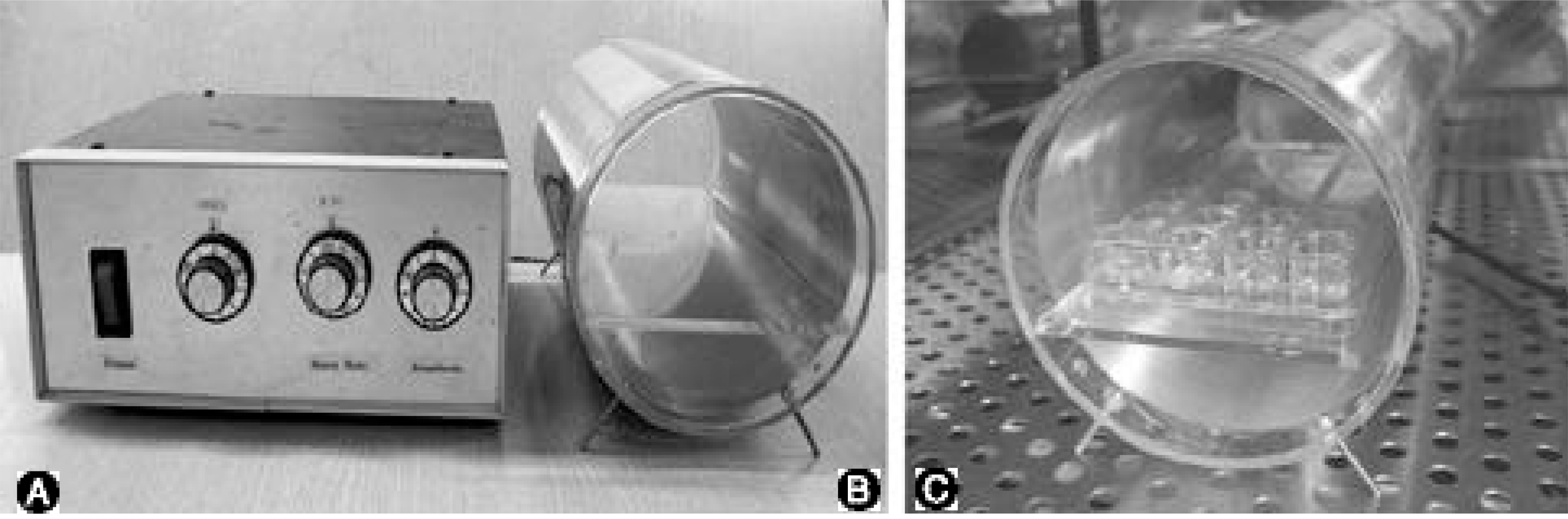
Fig. 2.
Percent control of DNA synthesis measured by [3H]-thymidine incorporation (CPM). Control; cultures without EMF exposure, EMF: cultures with EMF exposure for 6, 24, 48, 72 hours.
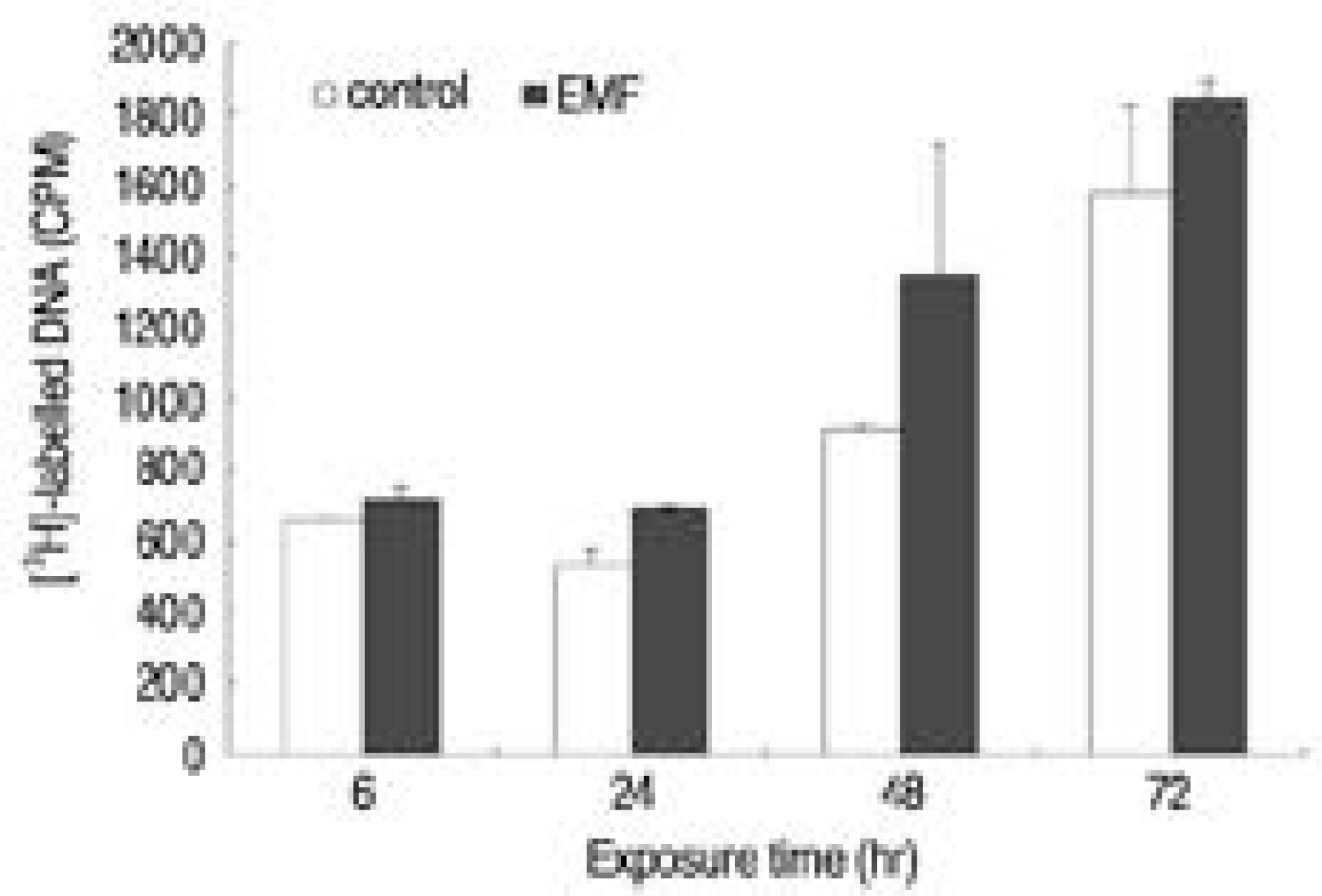
Fig. 3.
Percent control of proteoglycan synthesis measured by [35S]-Sulfate incorporation (CPM). Control: cultures without EMF exposure, EMF: cultures with EMF exposure for 6, 24, 48, 72 hours.
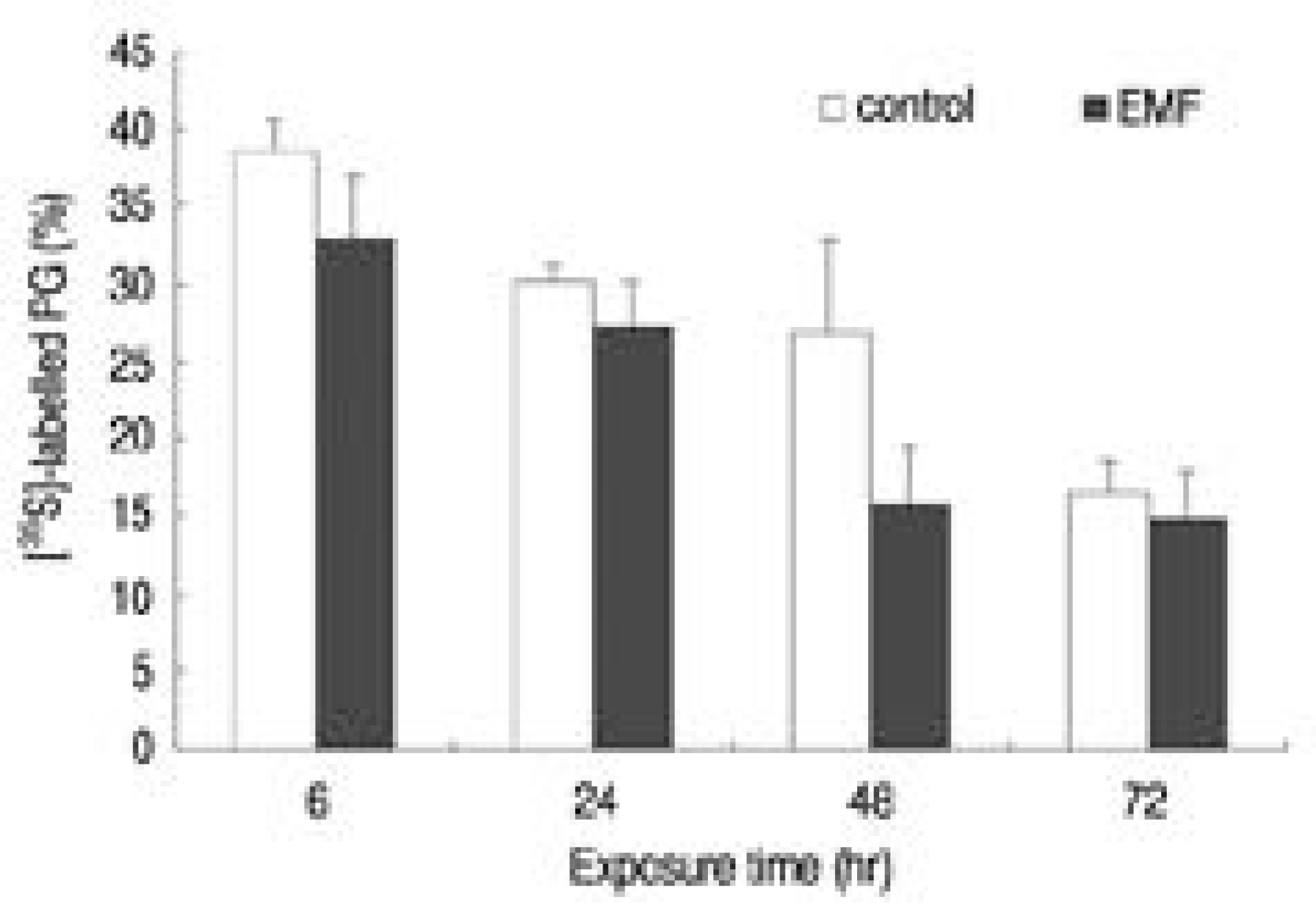
Fig. 4.
Effect of EMF on the expression of aggrecan and collagen type Ⅰ, Ⅱ. A; The IVD cells were exposed to various times of EMF. Total RNA was isolated from cells and subjected to RT-PCR. A; The PCR products were separated on 2% agarose gels containing ethidium bromide, and then observed on an ultraviolet transilluminator. B, C, D; The expression of each band seen in A was quantified using an image analyzer. The results are presented as the percentage of the mRNA level relative to β-actin for each band.
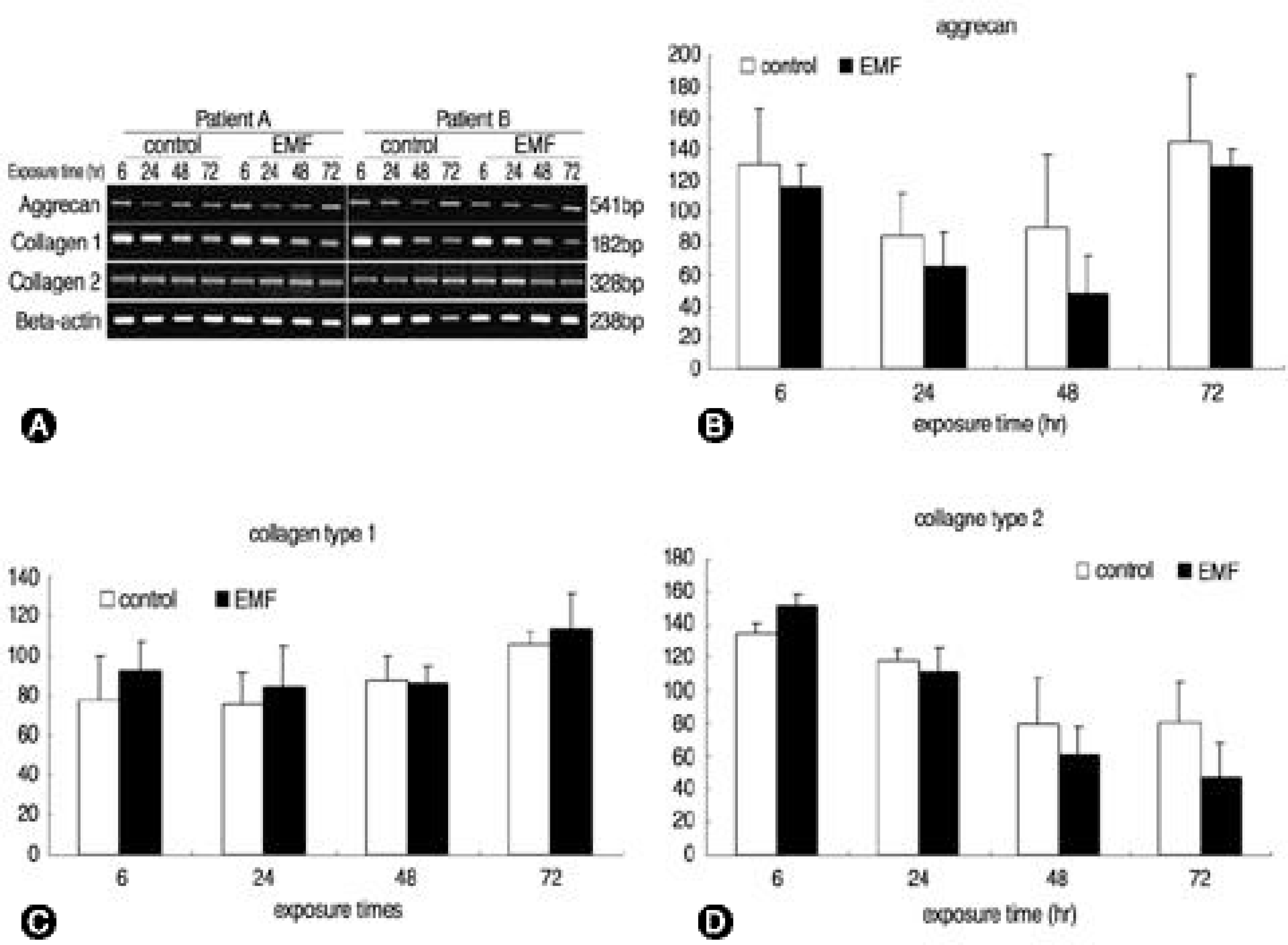
Table 1.
Sequences of the RT-PCR Primers Used




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download