Abstract
We report a case of a 23-year-old female immigrant from China who was diagnosed with multidrug-resistant tuberculosis affecting her lung and brain, resistant to the standard first-line therapeutics and streptomycin. She was treated with prothionamide, moxifloxacin, cycloserine, and kanamycin. However, her headache and brain lesion worsened. After the brain biopsy, the patient was confirmed with intracranial tuberculoma. Linezolid was added to intensify the treatment regimen, and steroid was added for the possibility of paradoxical response. Kanamycin was discontinued 6 months after initiation of the treatment; she was treated for 18 months with susceptible drugs and completely recovered. To our knowledge, this case is the first multidrug-resistant tuberculosis that disseminated to the brain in Korea.
Multidrug-resistant tuberculosis (MDR-TB) is defined by the infection with Mycobacterium tuberculosis resistant to at least two of the most effective anti-tuberculosis medications, isoniazid (INH) and rifampin (RFP). Extensively drug-resistant tuberculosis (XDR-TB) is defined as TB strains resistant to INH and RFP, and also resistant to any fluoroquinolone and at least 1 of 3 injectable second-line drugs; i.e., amikacin, kanamycin, or capreomycin. Fluoroquinolones and injectable drugs are the most effective second-line therapeutics; loss of one or both drugs can consequently weaken treatment regimens [12].
Rapid diagnosis and optimal selection of treatment regimen is essential for successful treatment of MDR-TB [12]. MDR-TB is a problem of increasing importance, especially in eastern Asia, including South Korea, where the TB prevalence rate is intermediate. For cases of MDR-TB with central nervous system (CNS) involvement, it is more difficult to select the optimal regimen. Here, we report what we believe to be the first case in Korea of an MDR-TB infection with multiple organ involvement including the CNS, confirmed by brain biopsy [13].
A 23-year-old female without any underlying disease presented to the emergency room with a 2-week history of fever and general weakness. She immigrated to South Korea from Qingdao, China 2 months ago. The patient was initially treated with antibiotics at a local clinic for community-acquired pneumonia. She was transferred to an 800-bed teaching hospital owing to dyspnea and persistent fever.
On presentation, the patient looked pale and malnourished (152 cm, 35 kg, body mass index 15 kg/m2) and had a temperature of 38°C, heart rate of 118/min, respiratory rate of 28/min, and blood pressure of 100/62 mmHg. Her breathing sounds were coarse with crackles on whole lung fields. The cervical lymph nodes were not palpable.
Laboratory evaluations revealed the following values: white blood cell (WBC) count of 6,760/mm3, with 87.0% neutrophils and 7.8% lymphocytes, platelet count of 84,000/mm3, hemoglobin of 8.8 g/dL, aspartate aminotransferase of 295 IU/L, alanine aminotransferase of 171 IU/L, albumin of 2.3 g/dL, and C-reactive protein level of 7.27 mg/dL. A test for antibody to the HIV was negative. Arterial blood gas analysis showed oxygen pressure level of 50.2 mmHg and 89% oxygen saturation. On chest X-ray, there was diffuse bilateral ground glass opacity (GGO) in both lungs, especially lower lungs (Fig. 1A). Chest computed tomography on the day of admission demonstrated nodular consolidation in right upper lobe, as well as diffuse bilateral GGO and multifocal infiltration in both lungs, which were thought to be atypical pneumonia or pulmonary hemorrhage (Fig. 2).
Levofloxacin and imipenem/cilastatin were empirically initiated for treatment of pneumonia. The Gram stain of sputum showed many WBCs, few epithelial cells, and only some Gram-positive cocci, Gram-positive rods, Gram-negative rods.
On day 2 of admission, the patient was placed on a mechanical ventilator. No bacterium was cultured on sputum. Initial transtracheal aspirate specimen was negative by staining the acid-fast bacilli (AFB), but it was positive by tuberculosis polymerase chain reaction (TB-PCR, LG Lifescience, Seoul, Korea). On day 6 of admission, the patient was started the treatment with INH, RFP, etambutol (EMB), and pyrazinamide (PZA). Levofloxacin was also continued to treat possible combined bacterial pneumonia. On day 16 of admission, the patient's fever subsided.
After 26 days of admission, the culture of transtracheal aspirate on MGIT 960 medium yielded M. tuberculosis, was sent to the laboratory for rapid drug resistance assay and conventional drug sensitivity test of absolute concentration method by The Korean Institute of Tuberculosis [4]. On hospital day 31, rapid drug resistance assays by GenoType MTBDRplus turned out to be resistant to both INH and RFP with katG gene and rpoB gene mutation [5]. Administration of these drugs was stopped, and amikacin and moxifloxacin were added to the treatment regimen [12]. Chest radiography showed marked improvement (Fig. 1B). Meanwhile, on hospital day 34, she developed fever over 40℃ again, and a headache with increasing intensity. The cerebrospinal fluid (CSF) analysis showed white WBC count of 350/mm3 with 91% neutrophils and 8% lymphocytes, red blood cell count of 540/mm3, protein of 162 mg/dL, glucose of 35 mg/dL, and adenosine deaminase (ADA) level of 14.1 u/L. TB-PCR and AFB culture of CSF were negative. Magnetic resonance imaging of the brain showed high signal intensity on T2 weighted image with enhancement consistent with tuberculomas (Fig. 3A). After administration of the dexamethasone 0.4 mg/kg, the headache subsided. The follow up CSF was analyzed after 10 days, showing WBC count of 6/mm3, red blood cell count of 3/mm3, protein of 57 mg/dL, glucose of 44 mg/dL, and ADA of 13.5 u/L. The dexamethasone was tapered over 4 weeks.
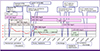
The result of conventional drug susceptibility testing was reported at the hospital day 71, which showed M. tuberculosis strain was resistant to RFP, INH, EMB, PZA, streptomycin and sensitive to amikacin, kanamycin, capreomycin, levofloxacin, moxicloxacin, ofloxacin, prothionamide, cycloserine, p-aminosalicylic acid and linezolid. Therefore, anti-TB medication was switched to kanamycin, prothionamide, cycloserine with moxifloxacin (Fig. 4).
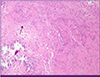
After 20 days of treatment with second line anti-TB medication, on hospital day 93, the headache reappeared, the frontal brain lesion enlarged and surrounding edema worsened in brain CT (Fig. 3B). The stereotactic brain biopsy was performed to confirm CNS TB for brain mass. The CSF analysis was normal except mildly elevated protein of 99 mg/dL. The CSF was negative for TB-PCR nor AFB culture. The biopsy showed only granulomatous necrotic tissue positive by TB-PCR, suggestive CNS-TB (Fig. 5). The brain tissue did not show any positive micro-organisms with Ziehl-Neelsen, Periodic acid-Schiff, Gomori's methanamine silver, and Gram stain. But culture for brain tissue was not performed. Sputum culture was negative since hospital day 22, there was no sign of pulmonary tuberculosis. The dexamethasone 10 mg/d was started for the possibility of paradoxical enlargement of intracranial tuberculoma. Linezolid was added to intensify the regimen, and the headache improved without neulorogic sequelae. Kanamycin was stopped after 6 month-treatment due to pain of injection site. She was treated for 18 months after initial TB diagnosis with susceptible drugs, and completely recovered. The chest X-ray was completely resolved (Fig. 1C) and the brain lesions on brain CT were stable without enhancing lesion (Fig. 3C).
The emergence of MDR-TB is one of the major concern about public health. Despite a complex treatment regimens and longer treatment periods, second-line anti-TB drugs are overall less effective than first-line drugs, which are associated with a higher incidence of adverse drug reactions [12]. According to data from the Korea Centers for Disease Control and Prevention, 49,532 patients were diagnosed with TB in South Korea in 2012. The 78.5% was newly diagnosed, and 3.1% was foreigners. In Korea, 2.4% of new cases was infected by the MDR-TB strain in 2012, which is a significant increase compared to 1.6% of new cases in 1994 [3567]. The incidence of MDR-TB in China was even higher, at 5.7% of new case in 2007. The China was reported as high MDR-TB burden country containing with other 11 countries in WHO [36]. The drug sensitivity tests are recommended to newly diagnosed patients, which should be analyzed in the TB cased from high prevalence area of MDR-TB [12].
WHO underscores that MDR-TB treatment regimens must include at least 4 effective drugs, typically first-group drugs, an injectable drug, a quinolone, and fourth or fifth-line drugs and to be continued for at least 24 months [2]. The optimal regimen to MDR-TB of CNS is not determined. Ethionamide, prothionamide, cycloserine, and fluoroquinolones such as moxifloxacin have sufficient penetration across the BBB to be useful for treatment of CNS-TB [18]. Moxifloxacin had been shown to be the most potent quinolone for tuberculosis in in vivo experiments, and easily crosses the BBB [128]. Prothionamide, and cycloserine were included in our regimen on the basis of both on their ability to cross the BBB and the drug resistance of the isolated bacterium [12910]. Linezolid was reported to be rapidly penetrate into CSF and considered for treatment of patients with CNS infection in previous studies [111213]. Furthermore, intracranial tuberculoma of this case was enlarged during the treatment, which was explained by two possibilities. The first reason is a paradoxical response which can be explained of an interaction between the host immunity and the direct effects of mycobacteria [14]. The second possibility was brain mass, other than tuberculoma, which was necessary to be verified by brain biopsy. After the brain lesion was confirmed to be a tuberculoma, we decided to treat patients with linezolid and steroid.
Linezolid, fifth-group TB drug, can be considered as treatment option for MDR-TB and XDR-TB cases. Recently, clinical trials of linezolid have shown good culture conversion rates of 73-87% in highly drug-resistant pulmonary tuberculosis [91013]. Therefore, linezolid was added to three drugs to this confirmed case of MDR-TB in CNS with steroid, even though the consensus of therapy for MDR-TB of CNS was not reached. Several studies have reported successful treatment of highly drug-resistant TB with linezolid and background drugs [91013].
Patients with MDR-TB meningitis have shown poor prognosis, based on the previous case reports and single case series [1516171819]. According to a report of MDR-TB meningitis in the United States, mortality rate of 26 MDR-TB meningitis patients was 73% [18]. In South Africa, a report of 30 MDR-TB meningitis patients showed 66.7% of mortality rate [19]. Detection of MDR-TB, isolating of pathogenic strain and performing conventional drug sensitivity test is important in the patient who came from highly prevalent area of MDR-TB due to the poor prognosis of CNS-TB. We report a disseminated MDR-TB case developed in immigrant from China, highly prevalent of MDR-TB, which was controlled with second line drugs including linezolid.
Figures and Tables
Figure 1
(A) Chest radiograph on admission. Bilateral ground glass opacity is visible. (B) After 2 months of anti-tuberculosis treatment, chest radiography shows no active lung lesions. (C) After 18 months of anti-tuberculosis treatment. No residual lesion.
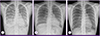
Figure 2
Computed tomography (CT) of chest at admission, showed diffuse bilateral ground glass opacity in both lungs and a nodular consolidation with pulmonary emphysema in right upper lung.
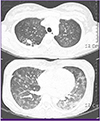
Figure 3
(A) Brain magnetic resonance image (MRI) on hospital day 31 showed brain edema and lesions on subcortical white matter of left frontal lobe and left temporal lobe which high signal intensity on T2-weighted image in the subcortical white matter of left frontal lobe (30×22 mm) and temporal lobe (24×20 mm), on coronal view (left) and transverse view (right). (B) Brain CT on hospital day 91. Ring like enhanced lesion with calcification in left frontal lobe (34×22 mm) and temporal lobe (28×19 mm) with enlargement of primary mass and progression of brain edema. (C) Brain CT After 20 months of anti-tuberculosis treatment, residual low density is in left frontal subcortical white matter without enhancement.
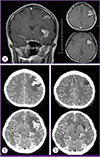
Figure 4
Clinical course of patients with disseminated MDR-tuberculosis.
MTB, Mycobacterium tuberculosis ; B-MRI, brain MRI; B-CT, brain CT; TB, tuberculosis; PCR, polymerase chain reaction; INH, isoniazid; RFP, rifampin; CXR, chest X ray; IPM, imipenem; LVX, levofloxacin; MXF, moxifloxacin; AMK, amikacin; KAN, kanamycin; EMB, ethambutol; CCS, cycloserine; PTH, prothionamide; DMS, dexamethasone; LZD, linezolid; HD, Hospital day.
References
1. Joint Committee for the Development of Korean Guidelines for Tuberculosis, and Korea Centers for Disease Control & Prevention. Korean Guidelines for Tuberculosis. 1st ed.2011. Accessed 10 July 2014. http://www.lungkorea.org/thesis/guide.php.
2. World Health Organization (WHO). Guidelines for the programmatic management of drug-resistant tuberculosis. 2011. Accessed 10 July 2014. Available at: http://www.lungkorea.org/thesis/guide.php.
3. Korea Centers for Disease Control and Prevention (KCDC). Annual report on the notified tuberculosis in Korea, 2012. Accessed 10 July 2014. Available at: http://www.cdc.go.kr/CDC/info/CdcKrInfo1001.jsp?menuIds=HOME001-MNU1155-MNU1083-MNU1375-MNU1084.
5. Lacoma A, Garcia-Sierra N, Prat C, Ruiz-Manzano J, Haba L, Rosés S, Maldonado J, Domínguez J. GenoType MTB-DRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol. 2008; 46:3660–3667.

6. World Health Organization (WHO). Global tuberculosis report, 2013. Accessed 10 July 2014. Available at: http://www.who.int/tb/publications/global_report/en.
7. Korea Centers for Disease Control and Prevention (KCDC). Current situation of MDR-TB and XDR-TB in Korea, 2009. Accessed 10 July 2014. Aveilable at: http://www.cdc.go.kr/CDC/contents/CdcK-rContentLink.jsp?fid=31&cid=12386&ctype=6.
8. Lee WY, Ling M, Anderson G, Achawal S, Thaker HK. Isoniazid-resistant intracranial tuberculoma treated with a combination of moxifloxacin and first-line anti-tuberculosis medication. J Med Microbiol. 2011; 60:1550–1552.

9. Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon HS, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee JK, Lee D, Kim CT, Dartois V, Park SK, Cho SN, Barry CE 3rd. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012; 367:1508–1518.

10. Chang KC, Yew WW, Tam CM, Leung CC. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother. 2013; 57:4097–4104.

11. Ntziora F, Falagas ME. Linezolid for the treatment of patients with central nervous system infection. Ann Pharmacother. 2007; 41:296–308.

12. Malacarne P, Viaggi B, DI Paolo A, Danesi R, Del Tacca M. Linezolid cerebrospinal fluid concentration in central nervous system infection. J Chemother. 2007; 19:90–93.

13. Schecter GF, Scott C, True L, Raftery A, Flood A, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010; 50:49–55.

14. Hawkey CR, Yap T, Pereira J, Moore DA, Davidson RN, Pasvol G, Kon OM, Wall RA, Wilkinson RJ. Characterization and management of paradoxical upgrading reactions in HIV-uninfected patients with lymph node tuberculosis. Clin Infect Dis. 2005; 40:1368–1371.

15. Joseph P, Desai VB, Mohan NS, Fredrick JS, Ramachandran R, Raman B, Wares F, Ramachandran R, Thomas A. Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India. Indian J Med Res. 2011; 133:529–534.
16. Kent SJ, Crowe SM, Yung A, Lucas CR, Mijch AM. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993; 17:987–994.

17. Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR Jr. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993; 328:527–532.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download