Abstract
Toxocariasis is a parasitic infection caused by the roundworms Toxocara canis or Toxocara cati, mostly due to accidental ingestion of embryonated eggs. Clinical manifestations vary and are classified as visceral larva migrans or ocular larva migrans according to the organs affected. Central nervous system involvement is an unusual complication. Here, we report a case of multiple cerebral infarction and concurrent multi-organ involvement due to T. canis infestation of a previous healthy 39-year-old male who was admitted for right leg weakness. After treatment with albendazole, the patient's clinical and laboratory results improved markedly.
Toxocariasis is a parasitic infection caused by infection with the roundworm species Toxocara canis or less frequently Toxocara cati whose hosts are dogs and cats, respectively [1]. Humans become infected accidentally by ingestion of embryonated eggs from contaminated soil or dirty hands, or by ingestion of raw organs containing encapsulated larvae [2]. Keeping dogs and ingestion of raw cow liver are associated with increased risk of toxoriasis. A cross-sectional study of Korean patients reported that a recent history of eating raw cow liver was associated with an increased risk of toxocariasis [3]. Clinical manifestation range from asymptomatic infection to fulminant disease, and the lungs, livers, and eyes are the most commonly involved organs [4]. Central nervous system (CNS) involvement is relatively rare in toxocariasis, especially CNS presenting as multiple cerebral infarction. We report a case of multiple cerebral infarction with lung and liver involvement due to T. canis infection in a previously healthy patient who was admitted for right leg weakness.
A 39-year-old right-handed man with no significant past medical history was admitted to the hospital with a 3-day history of right leg weakness. He had undergone discectomy for herniated nucleus pulposus at the L5-S1 level 8 years prior and had not experienced any complications. He smoked a pack of cigarettes per day for 20 years. He habitually ate undercooked meat, including 2 weeks prior to admission. He had no recent history of contact with pet animals and no past medical history of allergy.
On admission, his blood pressure was 120/80 mmHg, his pulse was regular at 88 beats per minute, and his temperature was 36.8℃. There were neither audible carotid bruits nor cardiac murmurs. Neurological examination revealed alert mental status and grade 3 weakness of the right leg. Deep tendon reflexes were increased in his right leg. Fundoscopy results were normal.
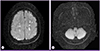
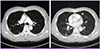
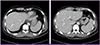
His white blood cell count was 11,900/mm3 with 26.7% eosinophils (2,600/mm3). Laboratory data were as follows: hemoglobin, 14.5 g/dL; platelets, 160,000/mm3; aspartate aminotransferase, 37 IU/L; alanine aminotransferase, 20 IU/L; alkaline phosphatase, 61 IU/L; creatinine 0.7 mg/dL; C-reactive protein, 32.8 mg/L; and erythrocyte sedimentation rate, 38 mm/h. Renal function and electrolytes were normal. The Venereal Disease Research Laboratory (VDRL) test and human immunodeficiency virus (HIV) antibody test in serum were negative. Electrocardiogram showed normal sinus rhythm and transthoracic echocardiogram showed no evidence of mural thrombi or vegetations. Diffusion-weighted brain MRI revealed multifocal small embolic acute infarctions in the internal border zone of both cerebral hemispheres, temporooccipital lobes of both PCA territory, and left cerebellar hemisphere (Fig. 1). Chest CT showed multiple peripheral ground glass opacities in both lungs that were considered eosinophilic pneumonia (Fig. 2). Abdominal CT on portal phase showed small ill-defined hypodense lesions in both lobes of the liver that were considered eosinophilic infiltrations (Fig. 3). His CSF contained 0/mm3 WBC, 63 mg/dL protein, and 49 mg/dL sugar; no bacteria or viruses were detected.
Because he had marked eosinophilia and multiple eosinophilic infiltrations in both lungs and liver, serologic testing for parasitic infections was performed. Serologic test result (performed at Seoul Clnical Laboratory (SCL), Seoul, Korea) for T. canis IgG antibody via enzyme-linked immunosorbent assay (ELISA, Bordier Affinity Products SA, Crissier, Switzerland) was positive with an antibody titer of 3.12 (reference range <1.00). This method has 91% sensitivity and 86% specificity [5]. Results of serologic tests for other helminthes (cysticercus, Paragonimus westermani, Clonorchis sinensis sparaganum, Entamoeba histolytica) were all negative. Any larva was not found in the CSF and CSF ELISA assay for T. canis IgG antibody was negative.
While biopsy could be performed to find Toxocara larva in the affected tissue and confirm the diagnosis, toxocariasis is usually diagnosed based on a combination of clinical symptoms, exposure history, marked eosinophilia, characteristic imaging findings and positive toxocariasis ELISA results. Thus, the patient was diagnosed with multiple cerebral infarction and concurrent multi-organ involvement due to T. canis infection without tissue biopsy. He was treated with albendazole (400 mg twice daily for 2 weeks) and steroid (prednisolone 30mg twice daily for 7 days). Prednisolone was gradually tapered for one month. On day 14 of albendazole administration, his leukocytosis was 14,300 /mm3 with 2.7% eosinophils. In subsequent weeks, the patient's leg weakness improved to grade 4. Two months after treatment, the follow-up tests including CT scan and brain MRI were planned but the patient didn't visit our hospital.
Toxocariasis is a zoonotic parasitic infection contracted from dogs and cats. This disease occurs in developed countries as well as in the tropics and sub-tropics where dog treatment and population control are limited [3]. Human infection occurs by ingestion of embryonated eggs in contaminated soil or on unwashed hands, therefore high exposure to dogs and cats and contaminated soil are associated with toxocariasis [2]. Occasionally, toxocariasis is caused by consumption of raw liver and meat contaminated with larvae of T. canis. In South Korea, chops of raw cow liver and meat are popular dishes and are thought to have health benefits; toxocariasis, including subclinical toxocariasis, is therefore prevalent in South Korea [36]. We report here a case of toxocariasis presenting as multiple embolic infarction with concurrent visceral migrans involving the lungs and liver. The patient frequently consumed raw cow liver and meat, and this was likely the source of infection.
Toxocara can infect any organ, but is commonly recognized in the lungs, liver, and eyes [4]. Although T. canis can cross the blood-brain barrier, CNS infection is rarely reported, but epilepsy, eosinophilic meningitis, meningo-encephalitis, encephalitis, arachnoiditis, vasculitis, meningo-myelitis, meningo-radiculitis, and optic neuritis due to Toxocara infection have been reported [78910]. Until recently, however, there has only been a few report of cerebral infarction due to toxocariasis [1112]. Toxocara larvae are metabolically active and produce an array of enzymes and waste products that cause tissue damage, necrosis, and a marked inflammatory reaction, with eosinophils as the major component [13]. The mechanism of cerebral infarction is not well known in toxocariasis, but could be derived from direct invasion of larva particles and secondary hypereosinophilia. Larva particles can accidentally invade the brain parenchyme or block cerebral vessels, similar to microemboli [14]. In hypereosinophilia, eosinophils could induce mural thrombus in the left ventricle or endomyocardial fibrosis by infiltrating the endocardium [15]. Direct eosinophilic toxicity to the vascular wall has also been hypothesized [16].
A definitive diagnosis of toxocariasis is based on finding larvae in the affected tissue by histologic examination. Clinically, the diagnosis is based on medical history, clinical presentation, eosinophilia in the serum, and/or high serum titer of T. canis antibodies as assessed by ELISA or western blotting [1]. Our patient had multiple cerebral infarctions concurrent with visceral migrans on CT, hypereosinophilia, positive serology for T. canis IgG, and a history of frequently eating raw cow liver and meat. He had no risk factors for multiple cerebral infarction and no cerebral vessel stenosis on cerebral CT angiography. We therefore diagnosed cerebral infarction caused by toxocariasis.
There is no proven effective therapy for patients with neurotoxocariasis due to the rarity of the disease. Albendazole is the most commonly used drug as it can penetrate the cerebrospinal fluid and has acceptable toxicity. Other antihelmintics include thiabendazole, mebendazole, oxibendazole, and flubendazole. Concurrent administration of corticosteroids has been used to suppress intense allergic responses [19]. In some studies, corticosteroids were effective at relieving serious neurologic symptoms brought on by an intensive inflammatory response [17] and increased plasma levels of albendazole by approximately 50% [18].
Because ingestion of uncooked cow liver and meat in South Korea is common, toxocariasis is prevalent. This infection has various clinical manifestations including asymptomatic eosinophilia and eosinophilic infiltration of the lungs, liver, and eyes. Central nervous system (CNS) involvement is relatively rare in toxocariasis, especially CNS presenting as multiple cerebral infarction. However, physicians should consider that cerebral infartion could occur simultaneously to the patient with toxocariasis.
Figures and Tables
References
1. Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16:265–272.

2. Chang S, Lim JH, Choi D, Park CK, Kwon NH, Cho SY, Choi DC. Hepatic visceral larva migrans of Toxocara canis: CT and sonographic findings. AJR Am J Roentgenol. 2006; 187:W622–W629.
3. Choi D, Lim JH, Choi DC, Lee KS, Paik SW, Kim SH, Choi YH, Huh S. Transmission of Toxocara canis via ingestion of raw cow liver: a cross-sectional study in healthy adults. Korean J Parasitol. 2012; 50:23–27.

4. Abdel Razek AA, Watcharakorn A, Castillo M. Parasitic diseases of the central nervous system. Neuroimaging Clin N Am. 2011; 21:815–841.

5. Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991; 29:1831–1835.

6. Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006; 85:233–238.

7. Dauriac-Le Masson V, Chochon F, Demeret S, Pierrot-Deseilligny C. Toxocara canis meningomyelitis. J Neurol. 2005; 252:1267–1268.

8. Duprez TP, Bigaignon G, Delgrange E, Desfontaines P, Hermans M, Vervoort T, Sindic CJ, Buysschaert M. MRI of cervical cord lesions and their resolution in Toxocara canis myelopathy. Neuroradiology. 1996; 38:792–795.

10. Liao CW, Cho WL, Kao TC, Su KE, Lin YH, Fan CK. Blood-brain barrier impairment with enhanced SP, NK-1R, GFAP and claudin-5 expressions in experimental cerebral toxocariasis. Parasite Immunol. 2008; 30:525–534.

11. Kim YB, Ko YC, Jeon SH, Park HM, Shin WC, Lee YB, Ha KS, Shin DJ, Lim YH, Ryu JS, Chung MS. Toxocariasis: an unusual cause of cerebral infarction. J Korean Neurol Assoc. 2003; 21:651–654.
12. Han WH, Kim JE, Do JK, Jung BW, Kwon HH. Cerebral infarctions associated with toxocariasis-induced secondary hypereosinophilia. Korean J Stroke. 2010; 12:109–111.
13. Xinou E, Lefkopoulos A, Gelagoti M, Drevelegas A, Diakou A, Milonas I, Dimitriadis AS. CT and MR imaging findings in cerebral toxocaral disease. AJNR Am J Neuroradiol. 2003; 24:714–718.
15. Grigoryan M, Geisler SD, St Louis EK, Baumbach GL, Davis PH. Cerebral arteriolar thromboembolism in idiopathic hypereosinophilic syndrome. Arch Neurol. 2009; 66:528–531.

16. Prick JJ, Gabreëls-Festen AA, Korten JJ, van der Wiel TW. Neurological manifestations of the hypereosinophilic syndrome. Clin Neurol Neurosurg. 1988; 90:269–273.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download