Abstract
Aspergillus tracheobronchitis (AT), an unusual form of invasive pulmonary aspergillosis (IPA), is characterized by pseudomembrane formation, ulcer or obstruction that is predominantly confined to tracheobronchial tree. Hematologic malignancies, neutropenia, solid organ transplantation, chronic corticosteroid therapy and acquired immunodeficiency syndrome (AIDS) are known to be major predisposing conditions. However, since the introduction of highly active antiretroviral therapy, there is only one reported case of AT in AIDS patient. After pandemic of influenza A/H1N1 2009, there are several reports of IPA in patient with influenza and most of them received corticosteroid or immunosuppressive therapy before the development of IPA. We present a 45 year-old AIDS patient with influenza A infection who developed pseudomembranous AT without corticosteroid use or immunosuppressive therapy.
Invasive pulmonary aspergillosis (IPA) is a serious and fatal disease, typically occurring in highly immunocompromised patients, which mainly involves the lung parenchyma [1]. Aspergillus tracheobronchitis (AT) is an unusual form of IPA, in which Aspergillus infection is confined entirely or predominantly to the tracheobronchial tree. AT is classified as pseudomembranous, ulcerative and obstructive, according to its bronchoscopic appearance [2]. As IPA, neutropenia, hematologic malignancies, solid organ transplantation (especially lung) or hematopoietic stem cell transplantation, and chronic corticosteroid therapy are main predisposing underlying conditions of AT. Advanced hunan immunodeficiency virus (HIV) disease, systemic lupus erythematosus, structural abnormalities of airway, uncontrolled diabetes mellitus and influenza are rare but possible predisposing factors of AT [2, 3].
AT is a rare condition in acquired immune deficiency syndrome (AIDS) patients. Its lower incidence among HIV-infected patients is associated with highly active antiretroviral therapy (HAART) [3, 4, 5]. After pandemic of influenza A/H1N1 2009, there are several reports of IPA in patient with influenza A [6]. But there is a few published report of AT in a HIV/AIDS patient with influenza A infection. Here, we report a 45 year-old AIDS patient with influenza A infection who developed pseudomembranous AT without corticosteroid use or immunosuppressive therapy.
A 45-year-old male was admitted with a productive cough, rhinorrhea, headache and fever over a period of 10 days on January 2013. On February 2012, he was diagnosed as HIV/AIDS, since then he was taking HARRT with lamivudine/abacavir and ritonavir-boosted atazanavir with good compliance. His initial CD4 T cell count and plasma HIV RNA level were 6 cells/mm3 and 203,863 copies/mL, respectively. The last CD4 T cell count was 87 cells/mm3 with undetectable HIV RNA load in plasma in November 2012. He was also taking sulfamethoxazole-trimethoprim for Pneumocystis jirovecii pneumonia (PJP) prophylaxis and oral hypoglycemic agents with subcutaneous insulin injection because of his diabetes. He had received valganciclovir from Februrary to December 2012 for CMV infection. He also diagnosed as diffuse large B cell lymphoma in May 2012, for which he had received six cycles of intravenous rituximab, cyclophosphamide, adriamycin, vincristine and prednisolone and 3 cycles of intrathecal methotrexate. He achieved complete remission in October 2012 and no recurrence was detected at last study which was performed just two weeks before his admission.
A week before admission, he came to outpatient clinic to check the result of his last study for lymphoma. He complained rhinorrhea, cough and headache without fever, but there were no new abnormalities on images of his neck, chest and abdominal computed tomography (CT), which were performed a week ago. His absolute neutrophil count (ANC) was 700/mm3. He was prescribed a single injection of granulocyte colony stimulating factor (G-CSF) and daily administration of oral levofloxacin.
On the day of admission, he came back to outpatient clinic because his symptoms were aggravated. Physical examination revealed an acutely ill appearance without fever and crackle with expiratory wheezing which was heard on his left lower lung field. White blood cell count was 2,100/mm3, ANC 670/mm3, hemoglobin 13.0 g/dL, platelet count 128,000/mm3, C-reactive protein 19.77 mg/dL and hemoglobin A1c was 6.2%. A new peribronchial infiltrate in left lower lung field was shown on his chest radiograph. We initiated cefepime 2 g per 8 hours with impression of community-acquired pneumonia and administered G-CSF until the recovery of neutropenia. PJP prophylaxis and HAART were continued. Because of no improvement, antibacterial agent was changed to imipenem on day 5 and vancomycin was added on day 9. No bacterium or fungus was recovered from initial and followed blood, urine and sputum culture. Oseltamivir 75 mg per 12 hours was added for 10 days from day 3 after the identification of influenza A virus from the patient's nasopharyngeal secretion by a real-time reverse transcription polymerase chain reaction.
Despite administration of broad-spectrum antibacterial and antiviral treatment and recovery from neutropenia, there was no clinical improvement. Rather, the patient complained of dyspnea and chest discomfort. Chest CT was done on day 12 and it revealed newly developed numerous centrilobular nodules and peribronchial consolidations (Fig. 1). In bronchoscopy, whitish thick mucus plugs were observed from vocal cord via trachea to whole bronchi, reported as highly suggestive of pseudomembranous AT (Fig. 2). Both bronchoalveolar lavage (BAL) analysis and pathologic examination of the biopsy specimens demonstrated invasive septate hyphae (Fig. 3). Cultures of BAL fluid yielded Aspergillus fumigatus. Two consecutive results of galactomannan (GM) test at two-day intervals were negative. Amphotericin B deoxyclolate was initiated (daily dose 1.0 mg/kg/day) but 3 days later the therapy was changed to voriconazole (6 mg/kg per 12 hours for first 24 hours, then 4 mg/kg per 12 hours) because of increased serum creatinine level and progression of disease. However, respiratory failure was developed on following day and the patient died (Fig. 4). Necropsy was not performed.
We conducted literature search in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and KoreaMed (http://www.koreamed.org) with the terms "Aspergillus tracheobronchitis", "tracheobronchial aspergillosis" or "bronchial aspergillosis" and "HIV" or "AIDS" to identify articles pertaining to the subject published between January 1985 and July 2013. We reviewed the bibliographies of the selected articles for additional relevant references. Finally, we found 11 cases of AT in AIDS patients in the literature since 1985 [3, 4, 7, 8, 9, 10] (Table 1). The predisposing factors were neutropenia, CD4 T-lymphocytopenia (< 100 cells/mm3), AIDS- defining opportunistic illnesses and corticosteroid use. Neutropenia was associated with drugs such as zidobudine, vincristine. Nine patients died but only five deaths were attributable to AT and subsequent development of invasive aspergillosis. All patient with pseudomembranous type died because of AT. A. fumigatus is the most common pathogen, accounting for 63%, followed by Aspergillus flavus and Aspergillus niger.
Advanced AIDS is known as one of the predisposing factors of IPA and AT [1]. However, the overall prevalence of IPA and AT in AIDS patients are less than 5% [3, 5]. Furthermore, with the introduction of HAART, the incidence of IPA and AT has been decreased markedly [3, 11]. There is no patient had AIDS among 12 cases of AT patients which were reported in South Korea from 1998 to 2010 [12]. In our review, there were only two cases since 2000 including our case. The patient who reported by Antinori et al, had started HAART at the time of diagnosis of AT [10]. This is the first case report of the patient who developed AT while receiving HAART.
The predisposing factors of AT were not differ from that reported in other forms of IPA in AIDS patients [4, 5]. CD4 T-lymphocytopenia, neutropenia and influenza A infection may act as possible predisposing factors of AT in this case. There is no evidence of lymphoma recurrence and his diabetes was well-controlled. Boots et al. described a case of postinfluenza pseudomembranous AT in a 35-year-old woman with CD4 T-lymphocytopenia (CD4+ count 16 cells/mm3) with poor mitogen responsiveness to Aspergillus [13]. Wauters et al [6]. reported nine IPA patients among 40 critically ill H1N1 patients at two tertiary hospitals in Belgium and most of IPA patients were received corticosteroid or immunosuppressive therapy before the development of IPA. The alteration of ciliary defences of airway has been proposed as the possible mechanism of development of AT [3] and it occurs during the early stages of influenza-derived pneumonia [14].
The clinical manifestation of AT is non-specific and variable. Cough, dyspnea and fever are the most common symptoms [3, 4, 7, 8, 9]. Wheezing or stridor was observed in about one-quarter of AT patients [3]. Bilateral or unilateral pulmonary infiltrates are the most common radiologic findings, but more than one-third of the reported cases displayed no abnormalities at admittance. The close follow-up of simple chest radiography in patients at high risk of IPA is important [1, 3, 15]. Chest CT is recommended with higher reliability compared to simple chest radiography (79.4% vs. 42.7%). Bronchoscopic examination with microbiologic sampling and culture of the biopsy specimens is the choice of diagnosis for AT. Histopathologic demonstration of Aspergillus-like hyphae invading the bronchial mucosa is the definitive diagnosis. The role of GM is not well established although the sensitivity of GM is 60% in overall AT patient [3]. In the majority of cases reviewed in this report, GM was not checked. In our case, the results of two serial serum GM after diagnosis of AT by bronchoscopy were all negative.
Although AT has a relatively benign course than other forms of IPA, is fatal. The all-cause in-hospital mortality rate was about 40%, and most deaths were considered to be directly attributable to invasive aspergillosis. Neutropenia is independently associated with poor outcome and neutropenic patients are more likely to be diagnosed with pseudomembranous form [3]. Delayed treatment also has been proposed as contributable factor to worse prognosis [16].
There are no specific recommendations for the optimal therapy of AT. Itraconazole is an effective antifungal agent in our review [3, 4, 7, 8, 9, 10]. For IPA, the guidelines of the Infectious Diseases Society of America recommend voriconazole as the primary therapy, since this second-generation triazole has been demonstrated to improve survival and result in fewer severe side effects than Amphotericin B-containing drugs [17]. However, with the paucity of reported cases, it is not clear whether voriconazole is an effective first-line therapy for AT in overall patients and in AIDS patients [3]. There is only one known case in which voriconazole was used as first-line therapy, but it was effective only when combined with caspofungin [10]. Several authors have reported their successful experiences with adjuvant local therapy, such as amphotericin B aerosol or bronchoscopic removal of necrotic tissue [4, 7].
AT is an uncommon but, fatal disease in AIDS patients. Its clinical presentation includes fever and respiratory symptoms (cough, dyspnea or wheezing) but is non-specific, and the initial radiographic examination lacks sensitivity for early diagnosis. Neutropenia, CD4 T-lymphocytopenia, AIDS-defining opportunistic illnesses and corticosteroid use are known major risk factors of AT. Additionally, influenza infection may be the one of possible risk factors. If an AIDS patient who is at high risk of AT infected by influenza, clinicians should be aware of this entity. Prompt investigation of AT including chest CT and bronchoscopy, and consideration of starting empirical or preemptive antifungal therapy are necessary for refractory cases.
Figures and Tables
Figure 1
Chest CT of the patient on hospital day 13 shows numerous small centrilobular nodules and peribronchovascular consolidations on both lungs.
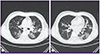
Figure 2
Bronchoscopic findings of the patient. Cream-colored thick pseudomembrane were found from vocal cord (A), carina (B), bronchus intermedius (C) to whole bronchi.
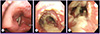
Figure 3
Microscopical findings of the specimen obtained from bronchoscopic biopsy and lavage show the numerous fungal hyphae invading the necrotic mucosa & submucosal layer, not the tracheal cartilage (A) (hematoxylin & eosin stain × 100) and tangled septate branching hyphae of Aspergillus species (B) (× 400), (C) (× 200), (D) (× 400).
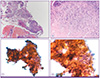
Figure 4
Clinical courses of the case patient according to hospital day (HD), with absolute neutrophil count (ANC), prescribed antibacterial, antiviral and antifungal agents, performed laboratory tests and the results of chest X-ray.
Tx, therapeutic methods; Dx, diagnostic methods; FEP, cefepime; IPM, imipenem; MEM, meropenem; VAN, vancomycin; OTV, oseltamivir; AMB, amphotericin B deoxyclolate, VRC, voriconazole; G-CSF, granulocyte colony stimulating factor; RT-PCR, real-time reverse transcription polymerase chain reaction for influenza A; CT, computed tomography; BR, bronchoscopy; BAL, broncho-alveolar lavage; CFP, cefepime; IMP, imipenem; MEP, meropenem; AMP-D, amphotericin B deoxycholate; G-CSF, ganulocyte colony-stimulating factor; RT-PCR, reverse transcription polymerase chain reaction for influenza A.
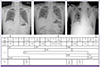
Table 1
Clinical characteristics, predisposing conditions and outcome in patients with AT and AIDS reported in the literature

Ref., reference; AT, aspergillus tracheobronchitis; AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; MAC, mycobacterium avium complex infection; CNS-Tox, central nervous system toxoplasmosis; PJP, pneumocystis jiroveci pneumonia; CMV, cytomegalovirus; CT, computed tomography; IC, itraconazole; AmB, Amphotericin B; VC, voriconazole; FC, flucytosine; Casp, caspofungin; w, weeks; d, days; iv, intravenous; Dx, diagnosis; NA, not available; A, Aspergillus.
aUnrelated death.
References
3. Fernández-Ruiz M, Silva JT, San-Juan R, de Dios B, García-Luján R, López-Medrano F, Lizasoain M, Aguado JM. Aspergillus tracheobronchitis: report of 8 cases and review of the literature. Medicine (Baltimore). 2012; 91:261–273.
4. Denning DW, Follansbee SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991; 324:654–662.

5. Holding KJ, Dworkin MS, Wan PC, Hanson DL, Klevens RM, Jones JL, Sullivan PS. Aspergillosis among people infected with human immunodeficiency virus: incidence and survival. Adult and Adolescent Spectrum of HIV Disease Project. Clin Infect Dis. 2000; 31:1253–1257.

6. Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R, Lagrou K, Wilmer A, Jorens P, Hermans G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012; 38:1761–1768.

7. Dal Conte I, Riva G, Obert R, Lucchini A, Bechis G, De Rosa G, Gioannini P. Tracheobronchial aspergillosis in a patient with AIDS treated with aerosolized amphotericin B combined with itraconazole. Mycoses. 1996; 39:371–374.

8. Kemper CA, Hostetler JS, Follansbee SE, Ruane P, Covington D, Leong SS, Deresinski SC, Stevens DA. Ulcerative and plaque-like tracheobronchitis due to infection with Aspergillus in patients with AIDS. Clin Infect Dis. 1993; 17:344–352.

9. Pervez NK, Kleinerman J, Kattan M, Freed JA, Harris MB, Rosen MJ, Schwartz IS. Pseudomembranous necrotizing bronchial aspergillosis. A variant of invasive aspergillosis in a patient with hemophilia and acquired immune deficiency syndrome. Am Rev Respir Dis. 1985; 131:961–963.
10. Antinori S, Ridolfo AL, Galimberti L, Milazzo L, Giuliani G, Ferraris L, Maruzzi M, Corbellino M. Usefulness of serial determination of Aspergillus galactomannan in the diagnosis and management of invasive aspergillosis in an AIDS patient with non-Hodgkin lymphoma. Mycoses. 2011; 54:e885–e888.

11. Hage CA, Goldman M, Wheat LJ. Mucosal and invasive fungal infections in HIV/AIDS. Eur J Med Res. 2002; 7:236–241.
12. Choi JK, No JH, Lee BH, Yun JS, Kim SH, Kwon JC, Hong JH, Lee GJ, Park SH, Choi SM, Lee DG, Choi JH, Yoo JH. Invasive tracheobronchial aspergillosis : case reports and a literature review. Infect Chemother. 2011; 43:76–81.

13. Boots RJ, Paterson DL, Allworth AM, Faoagali JL. Successful treatment of post-influenza pseudomembranous necrotising bronchial aspergillosis with liposomal amphotericin, inhaled amphotericin B, gamma interferon and GM-CSF. Thorax. 1999; 54:1047–1049.

14. Kim SH, Kim MN, Lee SO, Choi SH, Kim YS, Woo JH, Lim CM, Koh Y, Hong SB. Fatal pandemic influenza A/H1N1 infection complicated by probable invasive pulmonary aspergillosis. Mycoses. 2012; 55:189–192.

15. Tasci S, Glasmacher A, Lentini S, Tschubel K, Ewig S, Molitor E, Sauerbruch T, Lüderitz B, Rabe C. Pseudomembranous and obstructive Aspergillus tracheobronchitis - optimal diagnostic strategy and outcome. Mycoses. 2006; 49:37–42.

16. Thonar B, Yoder M, Cleaves C. Not your typical chronic obstructive pulmonary disease exacerbation: Aspergillus tracheobronchitis in a nonclassical immunocompromised host. South Med J. 2010; 103:361–365.

17. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008; 46:327–360.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download