Abstract
Infection-associated plasmacytosis is not uncommon; however, marked plasmacytosis in both peripheral blood and bone marrow that mimicks plasma cell leukemia is a very rare condition. We encountered a case of extreme plasmacytosis associated with Klebsiella pneumoniae sepsis in an aplastic anemia patient. A 42-year-old man presented with high fever of 5 days' duration. Hematological analysis revealed severe neutropenia and thrombocytopenia; his white blood cell count was 900/mm3, with 26% of plasma and plasmacytoid cells in peripheral blood. Bone marrow biopsy and aspiration showed 25% cellularity with marked plasmacytosis (80%), highly suggestive of plasma cell leukemia. On the eighth hospital day, K. pneumoniae was identified in blood and sputum cultures. Fever improved after switching antibiotics, although his hematological condition worsened. His bone marrow cellularity (plasma cell proportion) progressively decreased: the values were 25% (80%), 10% (26%), 10% (11%), and < 10% (< 4%) on the 8th, 30th, 60th, and 90th hospital day, respectively. His plasmacytosis was extremely severe but was confirmed to be reactive with polyclonality. The present case represents the first report of strong suspicion of K. pneumoniae sepsis-associated marked plasmacytosis in an aplastic anemia patient.
Marked plasmacytosis in peripheral blood and bone marrow (BM) is a rare condition, suggestive of plasma cell leukemia (PCL). However, extensive plasmacytosis has been described in various other plasma cell neoplasm-associated conditions, including bacterial sepsis, dengue fever, acquired immune deficiency syndrome (AIDS), drug eruption, and autoimmune disorders [1-6]. Aplastic anemia, characterized by decreased BM cellularity and pancytopenia, is associated with relative increases in plasma cells in BM but not in peripheral blood. Although rare, various infectious conditions may induce mild-to-moderate levels of plasmacytosis even in those with aplastic anemia.
Here, we present the first case of marked, transient plasmacytosis accompanying Klebsiella pneumoniae infection in an aplastic anemia patient. The patient's initial peripheral blood and BM findings were strongly suggestive of PCL but were proven to be reactive plasmacytosis via polyclonality observed by serum protein analysis and in BM immunohistochemical findings. Plasmacytosis gradually decreased, accompanied by negative conversion of blood cultures.
A 42-year-old man presented with high fever of 5 days' duration. He had been healthy without a noteworthy medical history and had not been in a tropical location in the recent past. On physical examination, he had an acute, ill appearance, a body temperature of 39.2℃, a pulse rate of 72 beats/minute, a blood pressure of 110/60 mm Hg, and no signs of tachypnea (22 respirations/minute). No remarkable erythroderma or lymphadenopathy was observed. There was no evidence of arthritis. No definite space-occupying lesion was noted on his neck, chest, abdomen, or pelvis by computed tomography (CT). A summary of his clinical course is illustrated in Figure 1.
Laboratory results on admission were as follows: hemoglobin level, 11.1 g/dL; white blood cell (WBC) count, 500/mm3; and platelet count, 6,000/mm3. Blood urea nitrogen (BUN; 20.1 mg/dL), creatinine (1.10 mg/dL), and calcium levels (8.4 mg/dL) were not increased. Although the alanine aminotransferase level (67 IU/L) was mildly elevated, the aspartate aminotransferase level (37 IU/L) was not. The C-reactive protein level was 44.4 mg/dL (reference range: 0.00-0.30 mg/dL). The serum immunoglobulin (Ig) and light chain measures were as follows: IgG, 22.88 g/L (reference range: 8.00-18.00 g/L); IgM, 1.08 g/L (0.038-2.460 g/L); IgA, 4.37 g/L (0.90-4.50 g/L); kappa light chain, 118.05 mg/L (3.30-19.40 mg/L); and lambda, 87.18 mg/L (5.71-26.30 mg/L). The rheumatoid factor was positive (18 IU/mL), but all the other autoimmune- related factors, including fluorescence anti-nuclear antibody, antineutrophil cytoplasmic antibody, and antibody panel for extractable nuclear antigen, were negative.
On chest radiography, pneumonic haziness was noted. This haziness worsened to definite pneumonic consolidation on the 4th hospital day (HD; Fig. 2A). Empirical intravenous antibiotic treatment with cefepime 2.0 g every 8 hours and ciprofloxacin 400 mg every 12 hours failed to improve his pneumonia or fever.
On the eighth HD, BM biopsy and aspiration showed 25% cellularity with marked plasmacytosis (80%), lymphoid cells (15%), and less than 5% of erythroid-myeloid-megakaryocytes. Immunohistochemical staining for kappa and lambda light chains on BM plasma cells demonstrated no light chain restriction (Fig. 1). The WBC count was 900/mm3, with 26% of plasma and plasmacytoid cells in peripheral blood. Cytogenetic analysis using the G-banding technique revealed a 46, XY karyotype without definite chromosomal aberration. Serum protein electrophoresis showed hypoalbuminemia and increased gamma-globulin with a polyclonal pattern. Serum protein immunoelectrophoresis and immunofixation electrophoresis showed no definite abnormal arc or band, respectively.
K. pneumoniae was identified in both blood and sputum cultures on the eighth HD. Antibiotic susceptibility testing demonstrated resistance to ampicillin and piperacillin, while the pathogen was negative for extended-spectrum β-lactamase production. Tests for other infectious pathogens including human immunodeficiency virus, hepatitis A, hepatitis B, hepatitis C, Epstein-Barr virus, aspergillus, syphilis, legionella, pneumococcus, and mycobacterium were negative. There was no evidence of acid fast bacilli infection by direct smear, culture, or polymerase chain reaction. Considering his continuous febrile neutropenia and isolation of K. pneumonia from blood, the antibiotics were changed on the eighth HD to vancomycin 1.0 g intravenously every 12 hours plus levofloxacin 500 mg every 24 hours; his fever started to subside (Fig. 1).
No evidence of pneumonia was observed on the chest radiograph obtained on the 15th HD (Fig. 2B). Follow-up blood cultures performed on the 15th and 20th HDs revealed no growth of bacteria (Fig. 1).
Body temperature, however, elevated again to 39.5℃ on the 20th HD. CT evaluation showed multiple necrotizing consolidations on both lungs, suggesting invasive fungal infection (Fig. 2C). A septated fungal organism was detected by CT-guided lung biopsy (Fig. 2D). Antibiotics were changed to meropenem 1.0 g every 8 hours, amikacin 1.0 g every 24 hours, and amphotericin B deoxycholate 1.0 mg/kg every 24 hours; his clinical course improved except for intermittent low-grade fever.
Follow-up BM biopsies and aspirations were performed on the 30th, 60th, and 90th HDs. These examinations revealed decreased cellularity (plasma cell proportion) of 10% (26%), 10% (11%), and < 10% (< 4%), respectively (Fig. 1).
Platelet concentrates and packed red blood cells were transfused, and granulocyte colony-stimulating factor (G-CSF) was administered several times starting from the second HD. However, severe pancytopenia, defined as < 200/mm3 of the absolute neutrophil count, did not improve. The patient underwent allogenic peripheral blood stem cell transplantation on the 98 HD. He died of pneumonia and intracranial hemorrhage 6 months after initial presentation.
Plasma cells are usually undetectable in peripheral blood. During bacterial infection, plasma cell populations can expand 10- to 60-fold in response to exogenous pathogens [7]. Due to very low baseline plasma cell numbers, even expansions of 60-fold usually fail to receive clinical attention. Rarely, extensive plasmacytosis mimicking PCL has been described in association with various infections, including Staphylococcus aureus, Pseudomonas aeruginosa, and Clostridium paraputrificum infections [4, 5, 8]. Altered immune conditions such as aortofemoral bypass [8], malignancy [4], drug and alcohol abuse with hepatitis C virus infection [1], or drug eruption [5] have been associated with bacterial infection-induced plasmacytosis.
K. pneumoniae infection is not uncommon in the clinical setting. However, to our knowledge, there has been no case of K. pneumoniae-associated severe plasmacytosis in an immunocompromised patient reported previously. Therefore, this is the first reported case of marked plasmacytosis mimicking PCL caused by K. pneumoniae infection in an aplastic anemia patient. The evidence for this assertion is as follows: (1) the plasmacytosis was accompanied by K. pneumoniae infection and gradually decreased after negative conversion of blood cultures; (2) there was no evidence of other infections (viral, bacterial, and fungal); (3) there was no evidence of known allergy; and (4) there was no evidence of autoimmune disease or malignancy.
Fungal pneumonia occurred on the 20th HD, however, the plasmacytosis did not rebound at that time. Furthermore, the patient had been aspergillus antigen-negative on the admission day, which suggests that the fungal infection and plasmacytosis were not causally related. Rheumatoid factor was positive; however, this finding was not considered clinically significant because there was no definite evidence of autoimmune-related antibodies or clinical symptoms.
We found only four cases of aplastic anemia with infection-associated plasmacytosis in the English literature (summarized in Table 1) [9-12]. In two cases of methimazol-induced aplastic anemia, aplasia and plasmacytosis were improved by treatment with broad-spectrum antibiotics, antifungal drugs, and glucocorticoids [9, 10]. It has been suggested that a lymphocyte-mediated immunogenic reaction, rather than cytotoxic factors, may have led to methimazol-induced hypoplastic BM, subsequently replaced by plasmacytosis [10]. In one patient with hepatitis-associated aplasia who died of septic monilial infection, systemic plasmacytosis was found during postmortem examination [11]. Mucormycosis has also been shown to induce plasmacytosis to levels up to 28% in peripheral blood [12]. The significance of plasma cell increments in aplastic anemia has yet to be elucidated. Collectively, these cases suggest that early diagnosis and treatment are important to improve clinical outcomes and that care should be taken not to misdiagnose patients as having plasma cell dyscrasia. Plasmacytosis at approximately 14 days after acute myeloid leukemia treatment may reflect the effectiveness of therapy and may imply a role for concurrent infection in the reactive plasmacytosis [13]. Relative plasmacytosis is not rare in aplastic anemia; however, a massive absolute increase in the numbers of plasma cells is very unusual, and its occurrence should be investigated to exclude multiple myeloma coexisting with hypoplasia of the BM [14]. In select cases, plasmacytosis may be a prodromal sign for underlying latent disease. In a pediatric case of BM aplasia and prominent plasma cell proliferation mimicking PCL, acute lymphoblastic leukemia later developed [15]. Therefore, regular follow-up examinations in pediatric patients are strongly recommended. In the present case, to identify and rule out the monoclonality of plasma cells, we performed extensive studies, including quantitative analysis of serum immunoglobulin, serum protein electrophoresis, immunofixation electrophoresis, serum kappa/lambda light chain quantitation, and immunohistochemical staining of BM sections; these investigations revealed polyclonal plasmacytosis. To find the underlying cause of our patient's polyclonal plasmacytosis, we conducted tests for viruses, bacteria, fungi, and mycobacteria, all of which were negative with the exception of the tests for K. pneumoniae in blood and sputum. In conclusion, this case report is the first to describe reactive plasmacytosis mimicking PCL caused by K. pneumoniae infection in an aplastic anemia patient.
Figures and Tables
Figure 1
Schematic diagram of the patient's clinical course.
The asterisk indicates the hospital day of bone marrow (BM) study; and inset, immunohistochemistry for kappa (left) and lambda (right). NG, no growth; PCs, plasma cells and plasmacytoid cells; CFPM, cefepime; CFX, ciprofloxacin; LFX, levofloxacin; VCM, vancomycin; MRPN, meropenem; AMK, amikacin; AMB, amphotericin B deoxycholate; H/E, hematoxylin and eosin.
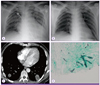
Figure 2
(A) Chest radiograph on the fourth hospital day (HD) shows pneumonic consolidation. (B) Chest radiograph on the 15th HD shows clear lung fields. (C) Computed tomography on the 20th HD shows multiple necrotizing consolidations on both lungs, suggesting invasive fungal infection. (D) Lung biopsy shows septated fungal hyphae (Gomori-methenamine silver stain, original magnification × 400).
References
1. Shtalrid M, Shvidel L, Vorst E. Polyclonal reactive peripheral blood plasmacytosis mimicking plasma cell leukemia in a patient with Staphylococcal sepsis. Leuk Lymphoma. 2003; 44:379–380.

2. Gawoski JM, Ooi WW. Dengue fever mimicking plasma cell leukemia. Arch Pathol Lab Med. 2003; 127:1026–1027.

3. Yamamoto M, Kumekawa H, Sasaki K, Murata Y, Ohki M, Kurosu T, Fukuda T, Arai A, Murakami N, Miura O. Marked reactive plasmacytosis accompanied by drug eruption in a patient with aplastic anemia. Rinsho Ketsueki. 2012; 53:526–530.
4. Peterson LC, Kueck B, Arthur DC, Dedeker K, Brunning RD. Systemic polyclonal immunoblastic proliferations. Cancer. 1988; 61:1350–1358.

5. Baker AM, Davis DW, Berg KK. Polyclonal systemic immunoblast proliferation: an unusual hematologic entity presenting as a medical examiner case. J Forensic Sci. 2001; 46:156–159.

6. Li L, Hsu P, Patel K, Saffari Y, Ashley I, Brody J. Polyclonal plasma cell proliferation with marked hypergammaglobulinemia and multiple autoantibodies. Ann Clin Lab Sci. 2006; 36:479–484.
7. Ten Boekel E, Siegert CE, Vrielink GJ, Van Dam VC, Ceelen A, De Kieviet W. Analyses of CD27++ plasma cells in peripheral blood from patients with bacterial infections and patients with serum antinuclear antibodies. J Clin Immunol. 2007; 27:467–476.

8. Poje EJ, Soori GS, Weisenburger DD. Systemic polyclonal B-immunoblastic proliferation with marked peripheral blood and bone marrow plasmacytosis. Am J Clin Pathol. 1992; 98:222–226.

9. Breier DV, Rendo P, Gonzalez J, Shilton G, Stivel M, Goldztein S. Massive plasmocytosis due to methimazole-induced bone marrow toxicity. Am J Hematol. 2001; 67:259–261.

10. Yamamoto A, Katayama Y, Tomiyama K, Hosoai H, Hirata F, Kimura F, Fujita K, Yasuda H. Methimazole-induced aplastic anemia caused by hypocellular bone marrow with plasmacytosis. Thyroid. 2004; 14:231–235.

11. Nishimoto Y, Iwahashi T, Nishihara T, Katayama H, Kuribayashi K, Takao T, Saito K. Hepatitis-associated aplastic anemia with systemic plasmacytosis. Acta Pathol Jpn. 1987; 37:155–166.

12. Munoz J, Hughes A, Guo Y. Mucormycosis-associated intracranial hemorrhage. Blood Coagul Fibrinolysis. 2013; 24:100–101.

13. Al-Shughair N, Al-Dawsari G, Gyger M, Mohamed G, Roberts G. Clinical significance of plasmacytosis in the day+14 bone marrow of patients with acute myeloid leukaemia undergoing induction chemotherapy. J Clin Pathol. 2007; 60:520–523.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download