Abstract
Purpose
The aim of this study was to evaluate the effect of immobilization of the recombinant human bone morphogenetic protein 2 (rhBMP-2) on anodized titaum implants coated with heparin to enhance the vertical alveolar ridge augmentation in the supraalveolar peri-implant defect region.
Materials and methods
18 pure titanium implants (7.0 mm in length, 3.5 mm in diameter) were manufactured for this study. All implants were anodized and designed insertion reference line marked with laser at the apical 2.5 mm from the fixture platform. Implantation of 6 noncoated anodized implants (Control group), 6 anodized implants physically adsorbed with rhBMP-2 by dip and dry method (BMP group) and 6 anodized implants chemically immobilized 3,4-dihydroxyphenylalanine (DOPA)-heparin/ rhBMP-2 (Hep-BMP group) was performed in the both mandibular of three male adult beagle dogs using split-mouth design. Radiologic examinations were performed immediately after implant placement and 4 and 8 weeks after implant placement. The amount of mesio-distal bone augmentation was evaluated by measuring the vertical distance from the platform to the marginal bone. Statistical analysis was performed using one-way analysis of variance (SPSS version 18.0) and multiple comparison analysis of The Kruskal-Wallis test and the Mann-Whitney U test. Statistical significance was established at the 5% significant level.
Results
At the 4 weeks vertical alveolar ridge augmentation of Control group, BMP group and Hep-BMP group is 0.09 ± 0.22 mm, 1.02 ± 0.72 mm, and 1.29 ± 0.51 mm, At the 8 weeks 0.11 ± 1.26 mm, 1.11 ± 0.58 mm, 1.59 ± 0.79 mm according to radiographic observations. The two experimental groups showed a significantly increasing in vertical bone height compared with the control group (P<.05). However, there is no significant difference between the BMP group and Hep-BMP group (P>.05).
REFERENCES
1.Junker R., Dimakis A., Thoneick M., Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009. 20:185–206.

2.Huh JB., Lee JY., Jeon YC., Shin SW., Ahn JS., Ryu JJ. Physical stability of arginine-glycine-aspartic acid peptide coated on anodized implants after installation. J Adv Prosthodont. 2013. 5:84–91.

3.Rammelt S., Heck C., Bernhardt R., Bierbaum S., Scharnweber D., Goebbels J., Ziegler J., Biewener A., Zwipp H. In vivo effects of coating loaded and unloaded Ti implants with collagen, chondroitin sulfate, and hydroxyapatite in the sheep tibia. J Orthop Res. 2007. 25:1052–61.

4.Hall J., Sorensen RG., Wozney JM., Wikesjo¨ UM. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J Clin Periodontol. 2007. 34:444–51.

5.Bessa PC., Casal M., Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts). J Tissue Eng Regen Med. 2008. 2:1–13.

6.Bessa PC., Casal M., Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2008. 2:81–96.

7.Mehta M., Schmidt-Bleek K., Duda GN., Mooney DJ. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 2012. 64:1257–76.

8.Boyne PJ., Marx RE., Nevins M., Triplett G., Lazaro E., Lilly LC., Alder M., Nummikoski P. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997. 17:11–25.
9.Hanisch O., Tatakis DN., Boskovic MM., Rohrer MD., Wikesjo¨ UM. Bone formation and reosseointegration in peri-implantitis defects following surgical implantation of rhBMP-2. Int J Oral Maxillofac Implants. 1997. 12:604–10.
10.Howell TH., Fiorellini J., Jones A., Alder M., Nummikoski P., Lazaro M., Lilly L., Cochran D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent. 1997. 17:124–39.
11.Sigurdsson TJ., Nygaard L., Tatakis DN., Fu E., Turek TJ., Jin L., Wozney JM., Wikesjo¨ UM. Periodontal repair in dogs: evaluation of rhBMP-2 carriers. Int J Periodontics Restorative Dent. 1996. 16:524–37.
12.Huh JB., Park CK., Kim SE., Shim KM., Choi KH., Kim SJ., Shim JS., Shin SW. Alveolar ridge augmentation using anodized implants coated with Escherichia coli-derived recombinant human bone morphogenetic protein 2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011. 112:42–9.

13.Stenport VF., Johansson C., Heo SJ., Aspenberg P., Albrektsson T. Titanium implants and BMP-7 in bone: an experimental model in the rabbit. J Mater Sci Mater Med. 2003. 14:247–54.
14.Schliephake H., Aref A., Scharnweber D., Bierbaum S., Roessler S., Sewing A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin Oral Implants Res. 2005. 16:563–9.

15.Stadlinger B., Pilling E., Huhle M., Mai R., Bierbaum S., Scharnweber D., Kuhlisch E., Loukota R., Eckelt U. Evaluation of osseointegration of dental implants coated with collagen, chondroitin sulphate and BMP-4: an animal study. Int J Oral Maxillofac Surg. 2008. 37:54–9.

16.Wikesjo¨ UM., Qahash M., Thomson RC., Cook AD., Rohrer MD., Wozney JM., Hardwick WR. Space-providing expanded polytetrafluoroethylene devices define alveolar augmentation at dental implants induced by recombinant human bone morphogenetic protein 2 in an absorbable collagen sponge carrier. Clin Implant Dent Relat Res. 2003. 5:112–23.
17.Kim J., Park Y., Tae G., Lee KB., Hwang CM., Hwang SJ., Kim IS., Noh I., Sun K. Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. J Biomed Mater Res A. 2009. 88:967–75.

18.Lee TC., Ho JT., Hung KS., Chen WF., Chung YH., Yang YL. Bone morphogenetic protein gene therapy using a fibrin scaffold for a rabbit spinal-fusion experiment. Neurosurgery. 2006. 58:373–80.

19.Gandhi NS., Mancera RL. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim Biophys Acta. 2012. 1824:1374–81.

20.Sasisekharan R., Ernst S., Venkataraman G. On the regulation of fibroblast growth factor activity by heparin-like glycosaminoglycans. Angiogenesis. 1997. 1:45–54.
21.Perets A., Baruch Y., Weisbuch F., Shoshany G., Neufeld G., Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003. 65:489–97.

22.Huh JB., Kim SE., Song SK., Yun MJ., Shim JS., Lee JY., Shin SW. The effect of immobilization of heparin and bone morphogenic protein-2 to bovine bone substitute on osteoblast-like cell's function. J Adv Prosthodont. 2011. 3:145–51.

23.Kim SE., Song SH., Yun YP., Choi BJ., Kwon IK., Bae MS., Moon HJ., Kwon YD. The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammation and osteoblast function. Biomaterials. 2011. 32:366–73.

24.Ishibe T., Goto T., Kodama T., Miyazaki T., Kobayashi S., Takahashi T. Bone formation on apatite-coated titanium with incorporated BMP-2/heparin in vivo. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 108:867–75.

25.Hong S., Kim KY., Wook HJ., Park SY., Lee KD., Lee DY., Lee H. Attenuation of the in vivo toxicity of biomaterials by poly-dopamine surface modification. Nanomedicine (Lond). 2011. 6:793–801.

26.Leknes KN., Yang J., Qahash M., Polimeni G., Susin C., Wikesjo¨ UM. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: radiographic observations. Clin Oral Implants Res. 2008. 19:1027–33.

27.Wikesjo¨ UM., Qahash M., Polimeni G., Susin C., Shanaman RH., Rohrer MD., Wozney JM., Hall J. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008. 35:1001–10.
28.Huh JB., Kim SE., Kim HE., Kang SS., Choi KH., Jeong CM., Lee JY., Shin SW. Effects of anodized implants coated with Escherichia coli-derived rhBMP-2 in beagle dogs. Int J Oral Maxillofac Surg. 2012. 41:1577–84.

29.Huh JB., Yun MJ., Jeong CM., Shin SW., Jeon YC. Combined effects of rhBMP-2 and rhVEGF coated onto implants on os-seointegration: pilot study. J Korean Acad Prosthodont. 2013. 51:82–9.

30.Boyne PJ., Lilly LC., Marx RE., Moy PK., Nevins M., Spagnoli DB., Triplett RG. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005. 63:1693–707.

31.Zhoua D., Ito Y. Inorganic material surfaces made bioactive by immobilizing growth factors for hard tissue engineering. RSC Adv. 2013. 3:11095–106.
32.Lee H., Scherer NF., Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci USA. 2006. 103:12999–3003.

33.Fan X., Lin L., Dalsin JL., Messersmith PB. Biomimetic anchor for surface-initiated polymerization from metal substrates. J Am Chem Soc. 2005. 127:15843–7.

34.Lee H., Dellatore SM., Miller WM., Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007. 318:426–30.

35.Lai M., Cai K., Zhao L., Chen X., Hou Y., Yang Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules. 2011. 12:1097–105.
36.Lee DW., Yun YP., Park K., Kim SE. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone. 2012. 50:974–82.

37.Kempen DH., Lu L., Hefferan TE., Creemers LB., Maran A., Classic KL., Dhert WJ., Yaszemski MJ. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008. 29:3245–52.

38.Kim SE., Yun YP., Lee JY., Shim JS., Park K., Huh JB. Co-delivery of platelet-derived growth factor (PDGF-BB) and bone morphogenic protein (BMP-2) coated onto heparinized titanium for improving osteoblast function and osteointegration. J Tissue Eng Regen Med. 2013 Jan 3.

39.Takada T., Katagiri T., Ifuku M., Morimura N., Kobayashi M., Hasegawa K., Ogamo A., Kamijo R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J Biol Chem. 2003. 278:43229–35.

40.Zhao B., Katagiri T., Toyoda H., Takada T., Yanai T., Fukuda T., Chung UI., Koike T., Takaoka K., Kamijo R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol Chem. 2006. 281:23246–53.

Fig. 1.
Schematic diagram for the immobilization of rhBMP-2 onto anodized Ti-implants coated with heparin and dopamine.
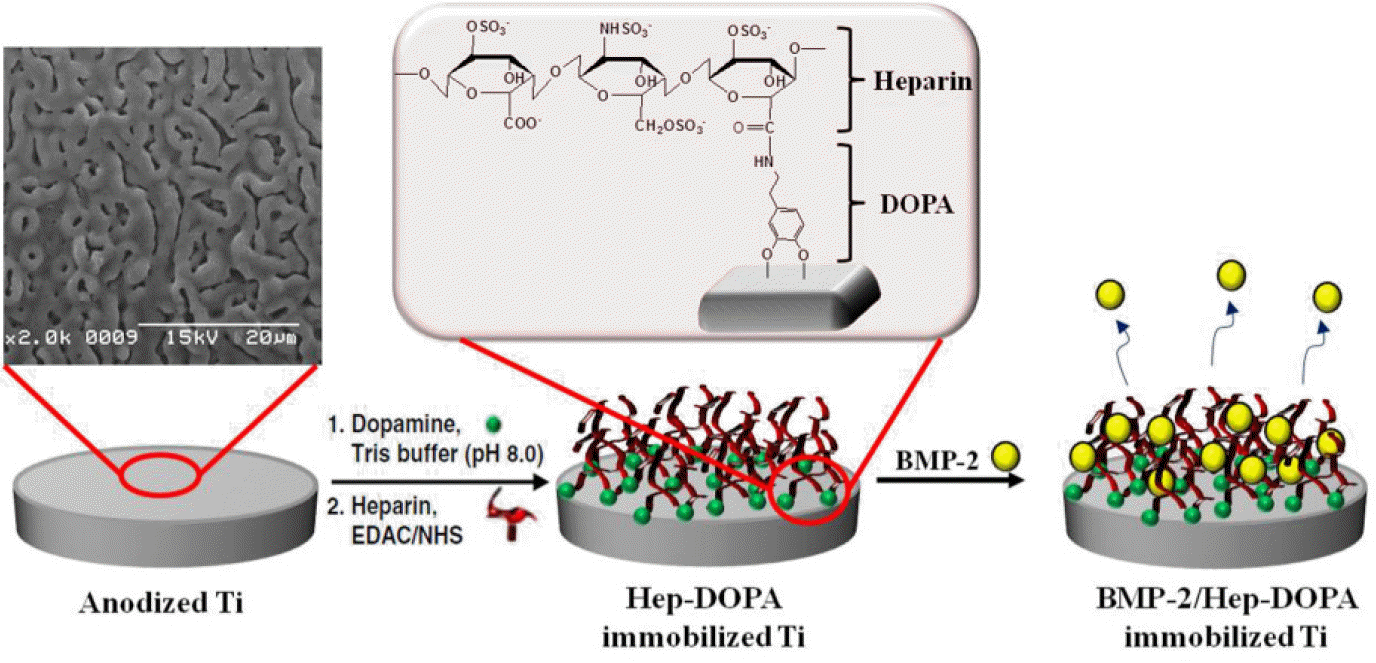
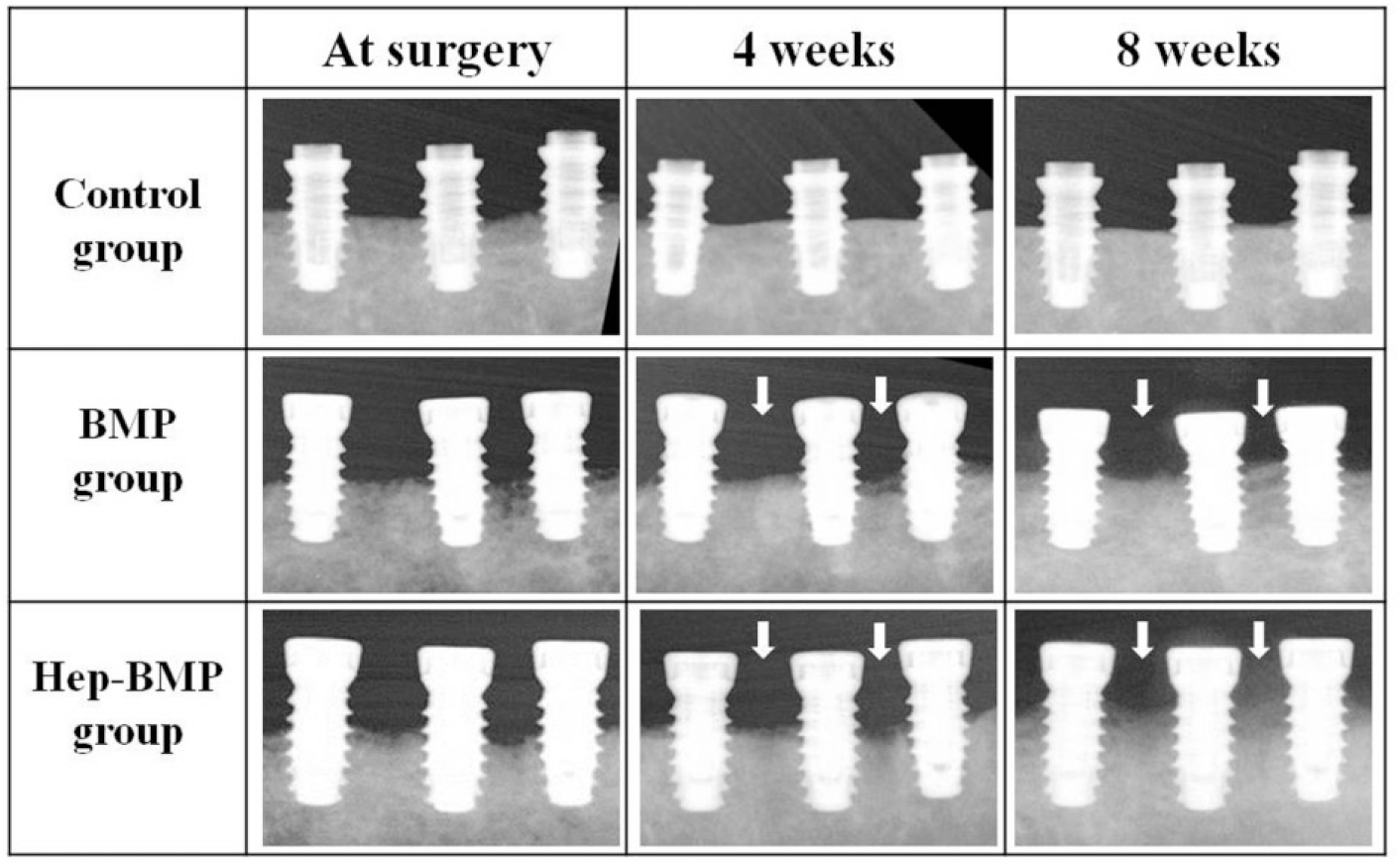
Fig. 2.
Radiographs of the mesio-distal bone height on peri-implant of two experimental groups and control group at 4 weeks and 8 weeks. The two experimental groups showed vertical bone gain at 4 and 8 weeks (arrow), whereas the control group did not show any changes.
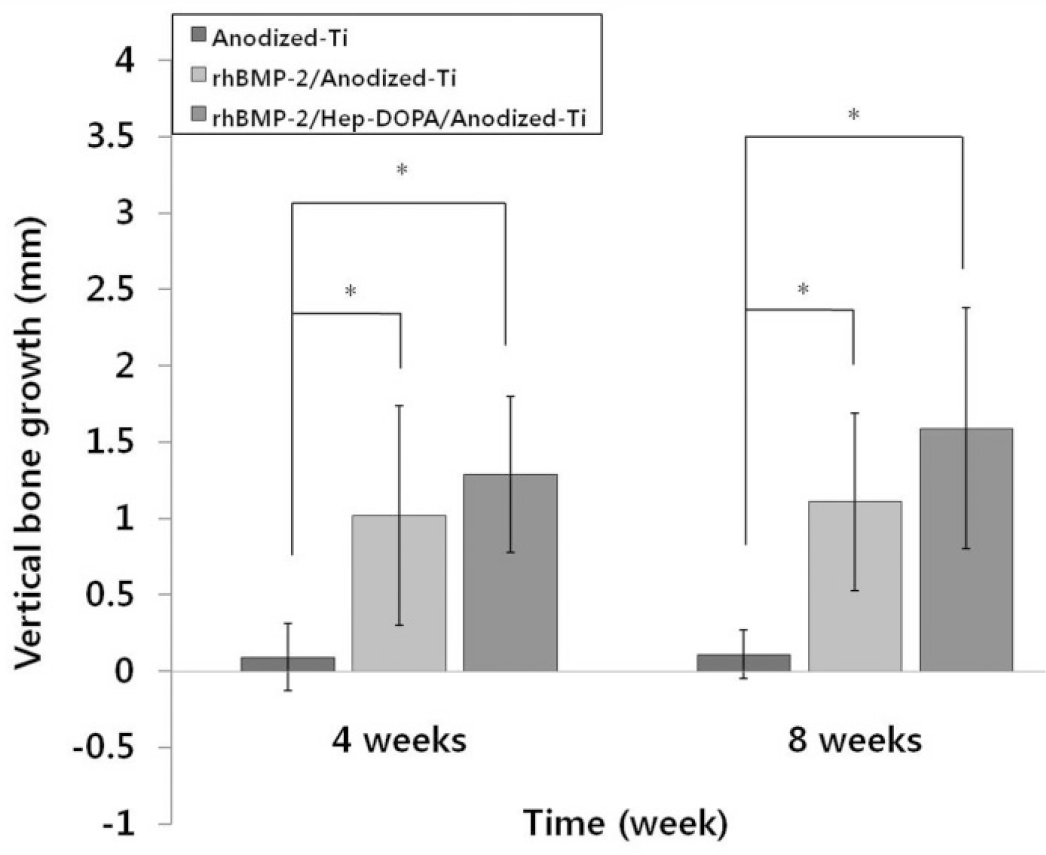
Fig. 3.
Mean (± SD) Graph of vertical bone growth on peri-implant at 4 weeks and 8 weeks. The BMP group and Hep-BMP group (experimental groups) showed increased vertical bone height. There is no statistically significant difference between the BMP group and Hep-BMP group, whereas the two experimental groups showed a significantly different increase in vertical bone growth compared with the control group (∗P<.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download