Abstract
Purpose
This study was aimed to evaluate the effect of rhPMP-2 coated implants on alveolar ridge augmentation in dogs.
Materials and methods
Six Beagle dogs were used in this study. Six 8.0 mm long anodized surface titanium implants were placed 5 mm into the mandibular alveolar ridge following 6 month of healing period after extraction. Each animal received three implants coated with rhBMP-2 and three uncoated control implants using the randomized split-mouth design. Radiographic examinations were undertaken i mmediately at implant placement (baseline), at weeks 4 and 8 after implant placement. The amount of bone augmentation was evaluated by measuring the distance from the uppermost point of the coverscrew to the marginal bone. Implant Stability Quotient (ISQ) values were measured i mmediately at implant placement and 8 weeks after implant placement. For the statistical analysis, Man-Whitney ranksum test and Wilcoxon signed rank test of SPSS 12.0 software were used (P = .05).
Results
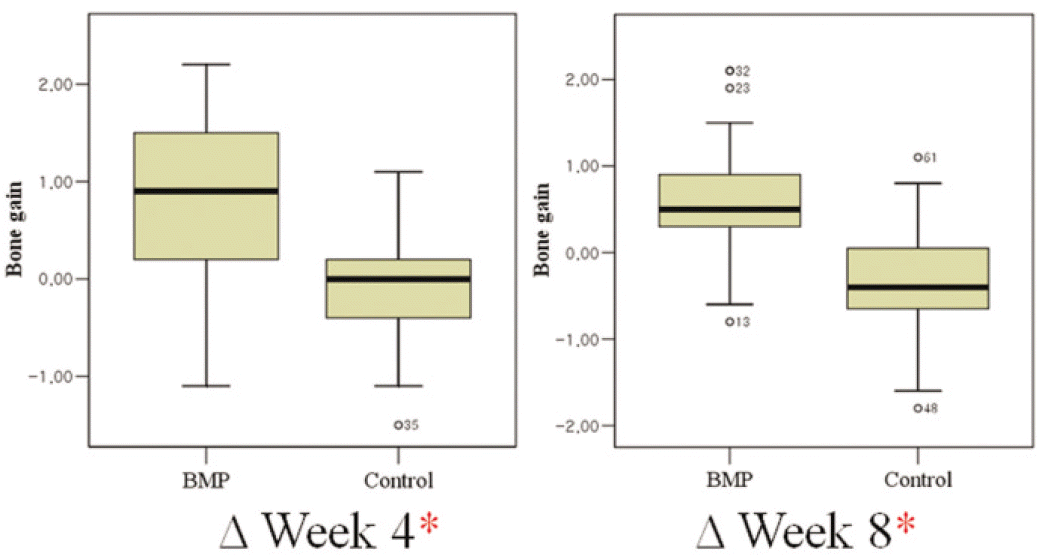
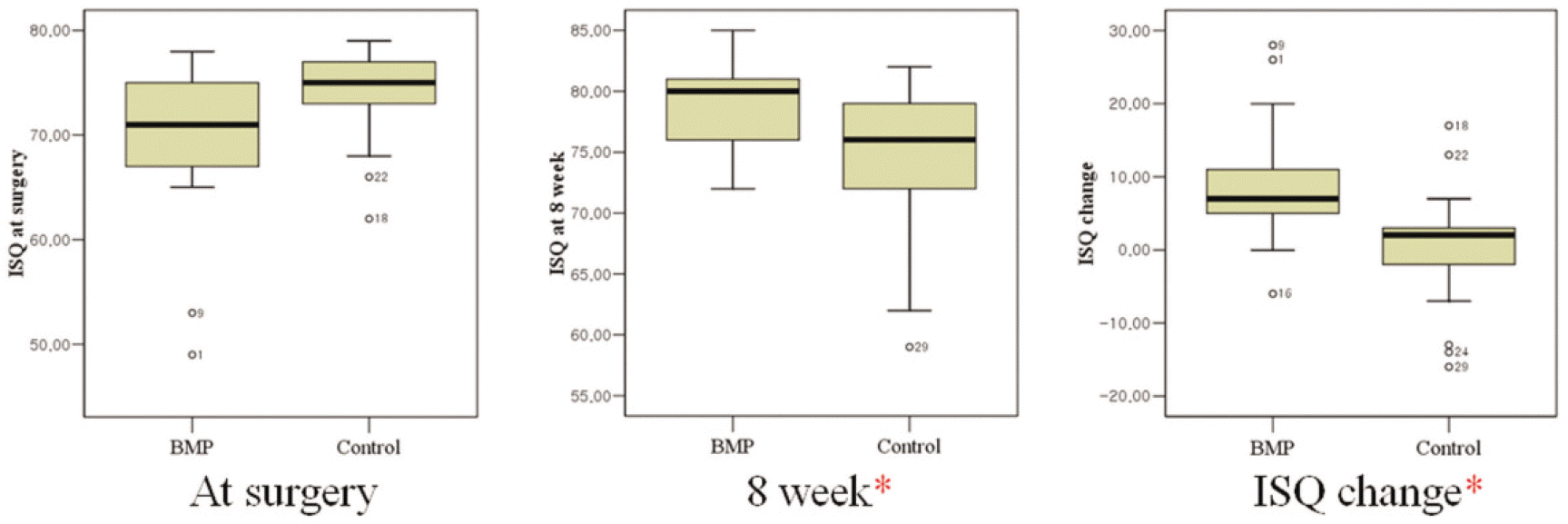
The BMP group exhibited radiographic vertical bone augmentation about 0.6 ± 0.7 mm at 8 weeks later while controls showed bone loss about 0.4 ± 0.6 mm. There was significant difference among the rhBMP-2 group and controls in bone level change (P < .05). The ISQ values were significantly higher in the BMP-2 group than the control group at 8 weeks later (P < .05), while there was no significant difference at surgery.
REFERENCES
3.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998. 16:247–52.

4.Bostrom MP., Asnis P. Transforming growth factor beta in fracture repair. Clin Orthop Relat Res. 1998. 355(Suppl):S124–31.

5.Barnes GL., Kostenuik PJ., Gerstenfeld LC., Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999. 14:1805–15.

6.Bessa PC., Casal M., Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts). J Tissue Eng Regen Med. 2008. 2:1–13.

7.Bessa PC., Casal M., Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J Tissue Eng Regen Med. 2008. 2:81–96.

9.Hall J., Sorensen RG., Wozney JM., Wikesjo ¨ UM. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J Clin Periodontol. 2007. 34:444–51.

10.Wikesjo ¨ UM., Xiropaidis AV., Qahash M., Lim WH., Sorensen RG., Rohrer MD., Wozney JM., Hall J. Bone formation at recombinant human bone morphogenetic protein-2-coated titanium implants in the posterior mandible (Type II bone) in dogs. J Clin Periodontol. 2008. 35:985–91.
11.Leknes KN., Yang J., Qahash M., Polimeni G., Susin C., Wikesjo ¨ UM. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: radiographic observations. Clin Oral Implants Res. 2008. 19:1027–33.

12.Simion M., Trisi P., Piattelli A. Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int J Periodontics Restorative Dent. 1994. 14:496–511.
13.Bessho K., Konishi Y., Kaihara S., Fujimura K., Okubo Y., Iizuka T. Bone induction by Escherichia coli - derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 2000. 38:645–9.
14.Hoar JE., Beck GH., Crawford EA., Resnik R. Prospective evaluation of crestal bone remodeling of a bone-density based dental implant system. Compend Contin Educ Dent. 1998. 19:17–24.
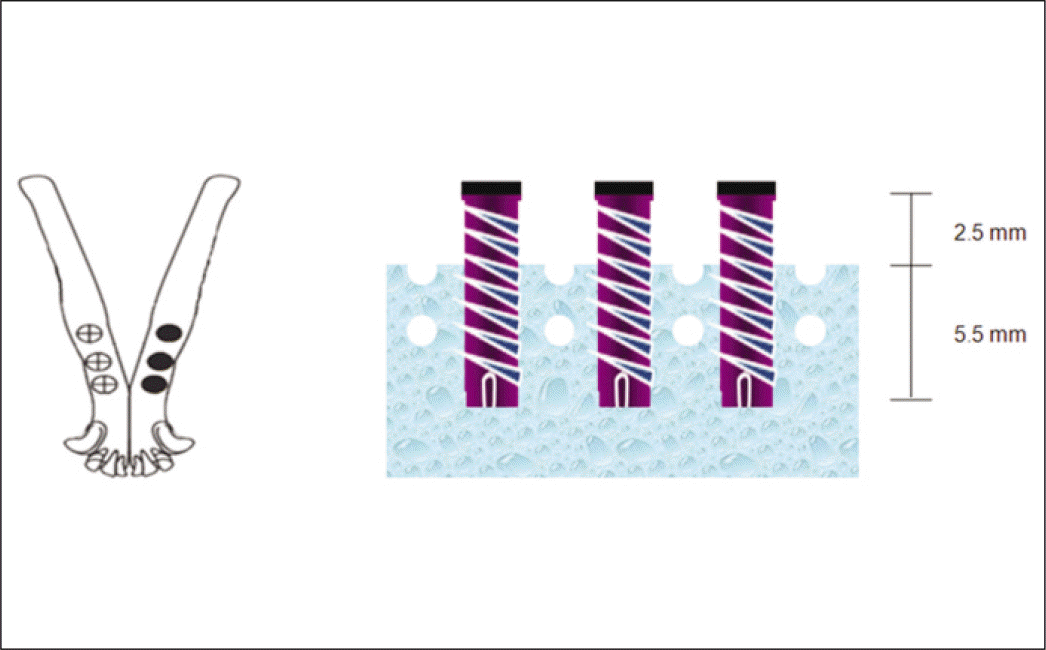
Fig. 1.
Design of experimental Implant (unit: mm). The experimental implants had internal connection, coronal microthread and macrothread.
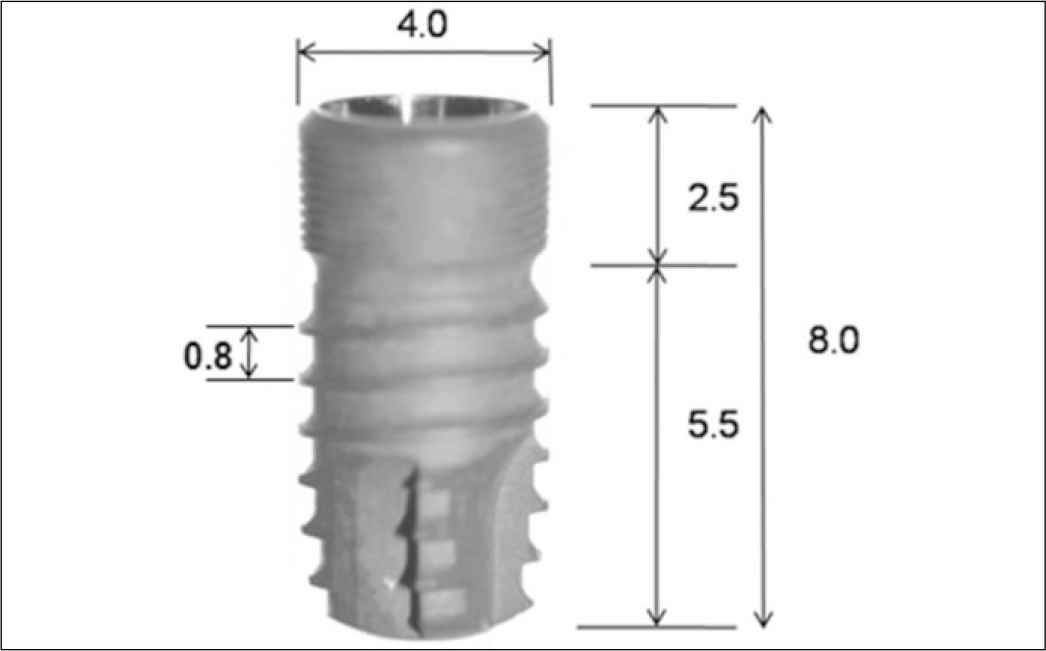
Fig. 3.
Implant surgery. A: Drilling, B: Implant placement, C: ISQ value measuring, D: Suture, E: 1 week after surgery, F: 8 week after surgery.
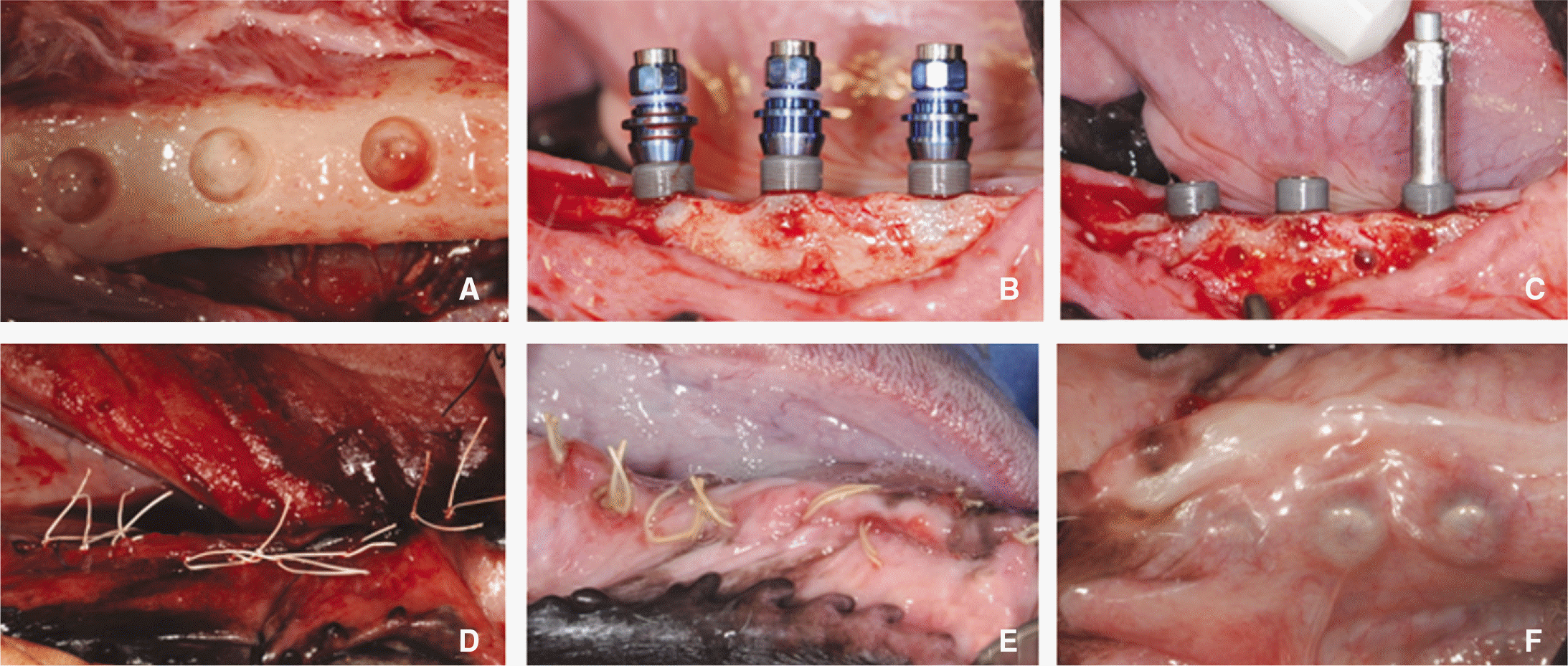
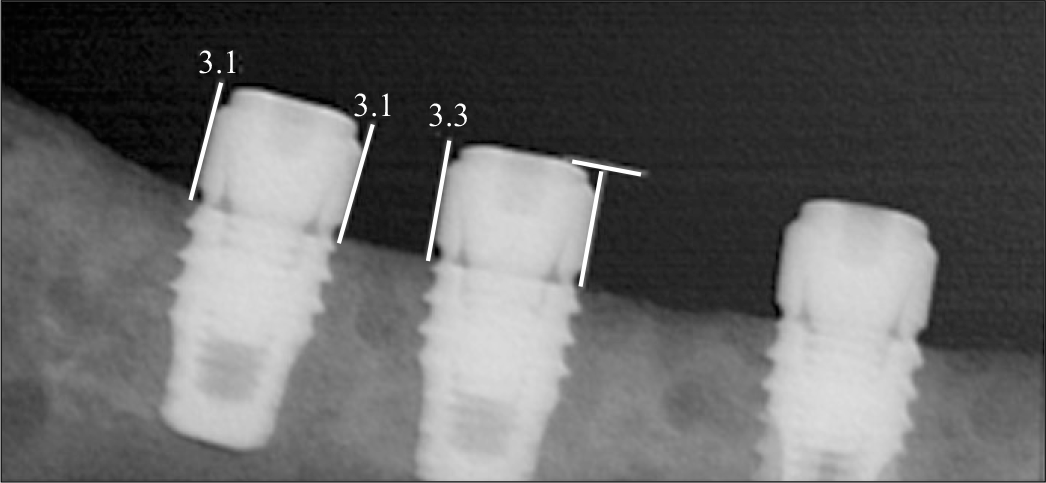
Fig. 6.
Periapical radiograph showing that implants coated with rhBMP-2 exhibited bone formation approaching the implant top, while there is no remarkable change of bone level on control implants.
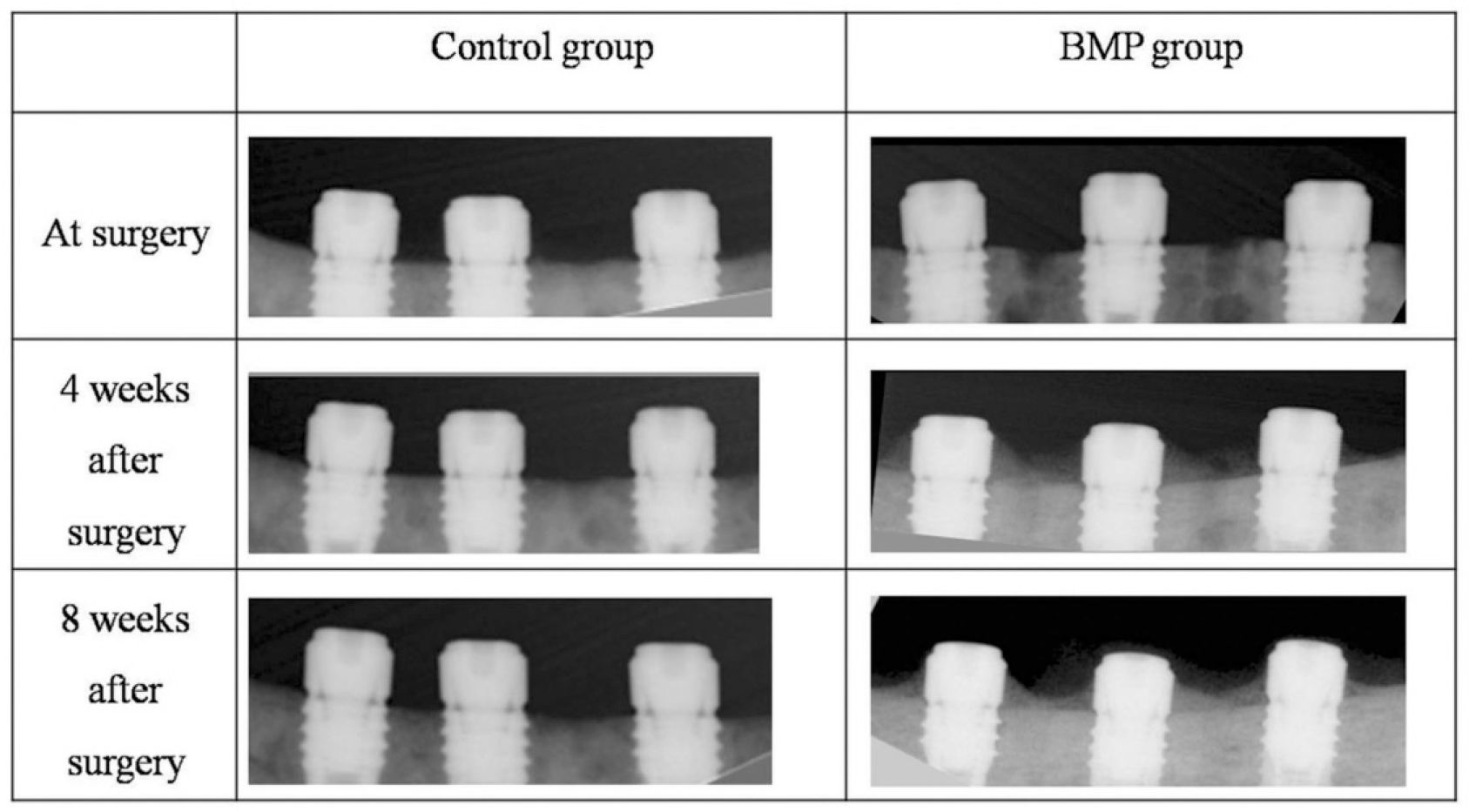
Fig. 9.
Failure of osseointegration of implant. A: At surgery, B: There is radiolucent halo on leftmost implant at 4 week after surgery, C: The radiolucent halo enlarged along almost all implant surface at 8 week after surgery.
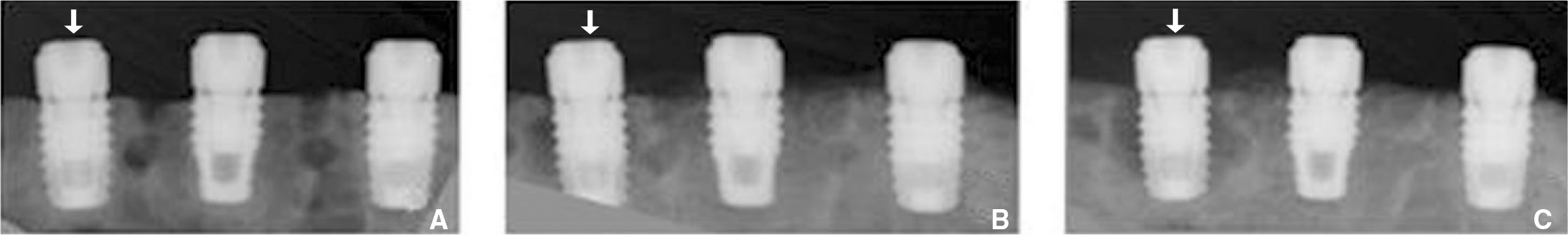
Fig. 10.
Exposure of implant fixture. A: Exposure of 3 implants of control group, B: Exposure of one implant of BMP group.
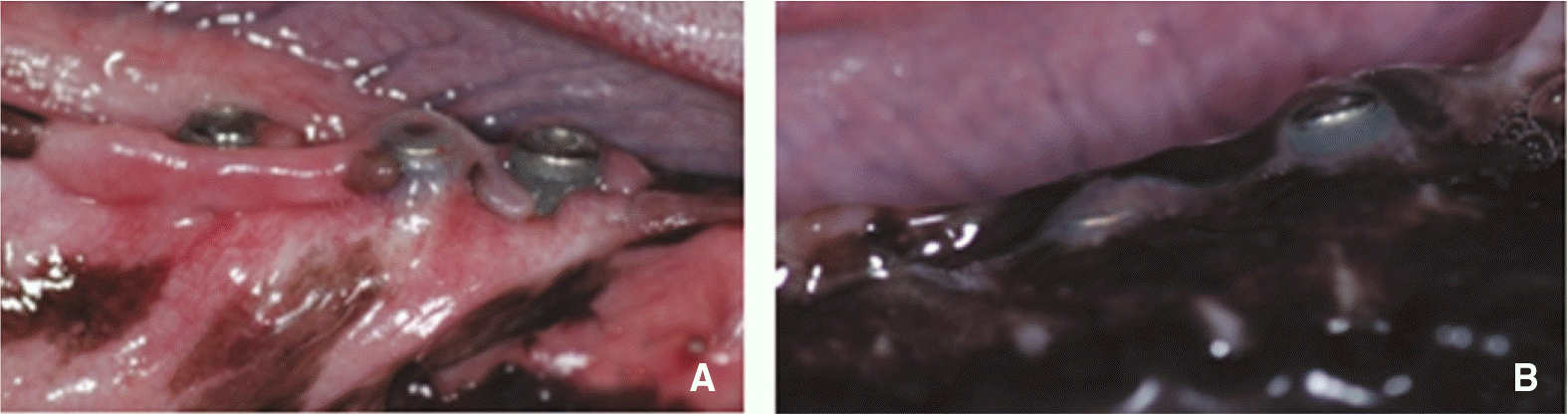
Table 1.
Numbers of experimental dogs and implants
| BMP group | Control group | |
|---|---|---|
| No. of implants | 3 | 3 |
| No. of dog | 6 | 6 |
| Total | 18 | 18 |
Table 2.
Mean (± SD) radiographic bone gain (mm) by group and observation interval
| ΔWeek 4 | ΔWeek 8 | Bone gain change | |
|---|---|---|---|
| BMP group | 0.85 ± 0.72 | 0.59 ± 0.69∗ | - 0.26 ± 0.47 |
| Control group | - 0.14 ± 0.52 | - 0.37 ± 0.62 | - 0.24 ± 0.47 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download