Abstract
We present the case of a 50-year-old male kidney transplant recipient whose verruca skin warts completely disappeared after conversion of immunosuppressive treatment from cyclosporine A to sirolimus. Before conversion, the patient underwent three sessions of cryotherapy, but this treatment failed to produce noticeable improvement in wart number or severity. In this case of a transplant recipient under CsA-based immunosuppression, change of immunosuppressive treatment to sirolimus produced dramatic improvement in the elimination of verruca warts.
Dermatological complications, such as, human papillomavirus (HPV)-induced warts, following transplantation are common in transplant recipients. In particular, warts are often recalcitrant to common treatments, and may become giant and painful, and thus, significantly impact quality of life(1). Furthermore, as duration of exposure to an immunosuppressive agent increases, the risks of these complications also increase(2), and are further increased by calcineurin inhibitors(3,4). Recently, sirolimus was introduced as a immunosuppressive, anti-malignancy, and anti-viral agent(5). Here, we describe the dramatic disappearance of verruca warts after initiating sirolimus in a renal transplant recipient.
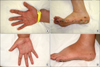
A 50-year-old renal transplant recipient complained of increasing numbers of skin warts on his hands and feet (Fig. 1A, B). Since kidney transplantation in 1994, he had been treated with cyclosporine A (CsA; Sansdimun Neoral, Novartis, Basel, Switzerland) and prednisolone (Solondo, Yuhan Medica, Cheongwon, Korea) for immunosuppression, and in 2002, he also started mycophenolic acid therapy (Myfortic, Novartis). Throughout this period, the CsA trough levels had been maintained at between 80 and 120 ng/mL. His renal function was stable, and his baseline creatinine level was around 1.5 mg/dL and had been well maintained before sirolimus conversion. His estimated glomerular filtration rate (eGFR; calculated by modification of diet in using the renal disease equation) remained at around 50~60 mL/min/1.73 m2 since kidney transplantation. He had experienced no acute rejection episode but 24-hour urine collection showed proteinuria at around 1,000 mg/day. The warts had grown slowly for 2 years from 2009, and in 2010, he was sent to the Department of Dermatology where he was diagnosed to have verruca vulgaris on both hands and feet, and underwent three sessions of cryotherapy. However, this failed to produce a noticeable improvement in wart number or severity. Because of this failure, sirolimus (Rapamune, Pfizer, New York, NY, USA) conversion was attempted from June, 2011. Sirolimus was loaded at 4 mg on the first day of conversion and followed by a maintenance dose of 2 mg/day. After starting sirolimus treatment, CsA was tapered by 25% weekly. The target trough level for sirolimus was 12~24 ng/mL. For 1 year after sirolimus conversion, no acute rejection episode occurred and serum creatinine levels and eGFR remained at acceptable levels (around 1.5 mg/dL and at 50~60 mL/min/1.73 m2, respectively). However, 1 month after starting sirolimus, the patient developed sirolimus induced interstitial pneumonitis, which was confirmed by bronchoscopy and chest computed tomography scan. At the time of diagnosis, his sirolimus blood levels were 18~24 ng/mL. The pneumonitis was relieved by reducing the dose of sirolimus to maintain sirolimus trough levels between 5 and 10 ng/mL. Interestingly, 4 months after starting sirolimus, the skin lesions dramatically disappeared (Fig. 1C, D). For 1 year after sirolimus conversion, his serum creatinine levels remained stable at around 1.5 mg/dL and serum sirolimus concentrations at around 5.0 ng/mL, but microprotein in 24-hour urine collection increased up to 2,500 mg/day.
Renal transplant recipients are predisposed to various dermatologic lesions, for example, HPV infections affect 6~92% of renal transplant recipients(6). Skin lesions can be associated with various skin infections, such as, viral, bacterial, or fungal infections, and viral-induced verruca vulgaris infection is apparently the most common skin infection in renal recipients(7).
In contrast to that observed in the healthy population, HPV-induced warts in transplant recipients may be resistant to common treatments, which mainly involve physical and/or chemical lesion destruction. Many transplant physicians have attempted to reduce immunosuppression in these patients but it has been considered as an insufficient method, and some case reports have mentioned the use of sirolimus for the treatment of warts in transplant recipients(8-10). Sirolimus is known to have an anti-proliferative effect, but little is known of the mechanism involved, and little information is available on the effect of sirolimus on HPV infections. On the other hand, the inhibition of virus replication by mammalian target of rapamycin inhibitors was recently investigated(11,12). In our case, we cannot exclude the possibility that CsA withdrawal contributed to wart regression. However, there was a report that sirolimus conversion showed successful result for the patients who was failed in the reduction of immunosuppression(10).
There can be some other treatment modalities. Bonatti et al.(13) reported effective response to local cidofovir in a series of six transplant recipients with HPV-associated skin lesions.
Sirolimus poses the risks of potentially fatal side effects, such as, pneumonitis, and has side effects that include proteinuria, hyperlipidemia, leg edema, and oral ulcer(14). Accordingly, close monitoring of sirolimus blood levels are required to minimize the risks of side effects(15). No definite indications are available for sirolimus conversion from a calcineurin inhibitor. However, the present case shows that sirolimus conversion can have a dramatic effect on verruca warts in transplant recipients under CsA-based immunosuppression.
Figures and Tables
References
1. Moloney FJ, Keane S, O'Kelly P, Conlon PJ, Murphy GM. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005. 153:574–578.

2. McLelland J, Rees A, Williams G, Chu T. The incidence of immunosuppression-related skin disease in long-term transplant patients. Transplantation. 1988. 46:871–874.

3. Pruvost C, Penso-Assathiany D, Bachot N, Lang P, Roujeau JC. Risk factors for cutaneous wart onset in transplant recipients. Ann Dermatol Venereol. 2002. 129:291–293.
4. Barba A, Tessari G, Talamini G, Chieregato GC. Analysis of risk factors for cutaneous warts in renal transplant recipients. Nephron. 1997. 77:422–426.

5. Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009. 69:2227–2243.
6. Hampton T. Skin cancer's ranks rise: immunosuppression to blame. JAMA. 2005. 294:1476–1480.
7. Serdar ZA, Eren PA, Canbakan M, Turan K, Tellioglu G, Gülle S, et al. Dermatologic findings in renal transplant recipients: possible effects of immunosuppression regimen and p53 mutations. Transplant Proc. 2010. 42:2538–2541.

8. Kostaki M, Venetz JP, Nseir G, Meylan P, Sahli R, Pascual M, et al. Giant warts in a kidney transplant patient: regression with sirolimus. Br J Dermatol. 2010. 162:1148–1150.

9. Shahidi S, Moeinzadeh F, Mohammadi M, Gholamrezaei A. Sirolimus-based immunosuppression for treatment of cutaneous warts in kidney transplant recipients. Iran J Kidney Dis. 2011. 5:351–353.
10. Dharancy S, Catteau B, Mortier L, Boleslawski E, Declerck N, Canva V, et al. Conversion to sirolimus: a useful strategy for recalcitrant cutaneous viral warts in liver transplant recipient. Liver Transpl. 2006. 12:1883–1887.

11. Di Benedetto F, Di Sandro S, De Ruvo N, Montalti R, Ballarin R, Guerrini GP, et al. First report on a series of HIV patients undergoing rapamycin monotherapy after liver transplantation. Transplantation. 2010. 89:733–738.

12. Gallego R, Henriquez F, Oliva E, Camacho R, Hernández R, Hortal L, et al. Switching to sirolimus in renal transplant recipients with hepatitis C virus: a safe option. Transplant Proc. 2009. 41:2334–2336.

13. Bonatti H, Aigner F, De Clercq E, Boesmueller C, Widschwendner A, Larcher C, et al. Local administration of cidofovir for human papilloma virus associated skin lesions in transplant recipients. Transpl Int. 2007. 20:238–246.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download