Abstract
Two young dogs were referred to the Veterinary Medical Teaching Hospital of Konkuk University, one for examination of vaginal discharge and the other after being hit by a car. Dog 1 exhibited a high neutrophil count on Gram-stained vaginal smears, marked leukocytosis on a complete blood count, and uterine enlargement on ultrasonography. In dog 2, a markedly enlarged right uterine horn containing echogenic debris was found incidentally on ultrasonography. A tentative diagnosis of pyometra was made in both cases and ovariohysterectomy was performed. Purulent material was collected from each uterine horn and submitted separately for aerobic and anaerobic bacterial culture; all culture results were negative. The white blood cell count revealed normal limits 2 days post operation in dog 1 and 4 days post operation in dog 2. Positive bacterial cultures are usually obtained from dogs with pyometra, and antibiotic selection is based on the results of culture and sensitivity testing in the event of failure of empiric antibiotic therapy. However, in the cases reported here, no bacterial growth was identified from the uterine samples despite the presence of purulent material. A short course of empiric antibiotic therapy was administered. This is the first known report describing sterile pyometra in dogs.
Pyometra is one of the most common diseases affecting intact bitches and is usually seen in middle or older age dogs (1). It can be a life-threatening condition since chronic and extreme inflammation is known to be associated with a sepsis-like disorder termed systemic inflammatory response syndrome (2). A combination of surgical ovariohysterectomy and antimicrobial drug therapy is considered to be the most effective treatment, and also prevents recurrence (23). Antimicrobial drugs can be selected on the basis of experience; however, samples for bacterial culture are necessary for antimicrobial susceptibility testing in the event of resistance to therapy (34). To the best of our knowledge, sterile pyometra has only been described in humans (5). The purpose of this case report was to introduce the term sterile pyometra and to describe the clinical presentation, diagnosis, and successful management of sterile pyometra in young dogs. This is the first known report describing sterile pyometra in dogs.
A 21 kg, 1-year-old, intact female Jindo dog and a 6 kg, 10-month-old, intact female Schnauzer were presented to the Veterinary Medical Teaching Hospital of Konkuk University for evaluation of vaginal discharge of two days’ duration and after being hit by a car, respectively. The owner of dog 1 reported that she had a history of twice-yearly estrus and mating during every estrus.
On physical examination of dog 1, yellowish vaginal discharge and a normal body temperature were noted. In dog 2, epistaxis, a gingival laceration, and normal ambulation were observed with normal body temperature. In dog 1, a high neutrophil count was identified on Gram-stained vaginal smears and laboratory tests revealed marked leukocytosis (62.74×103 cells/µL; reference range 6 to 17×103 cells/µL), a high level of C-reactive protein (56 mg/L; reference range 0 to 35 mg/L), elevations of alkaline phosphatase (167 U/L; reference range 15 to 127 U/L) and lactate dehydrogenase (299 U/L; reference range 0 to 133 U/L), and a low albumin concentration (2.0 g/dL; reference range 2.9 to 4.2 g/dL). In dog 2, no significant abnormalities were noted on a complete blood count. Serum chemistry analysis revealed elevations of alanine transaminase (1844 U/L; reference range 10 to 100 U/L), aspartate transaminase (1408 U/L; reference range 0 to 50 U/L), creatine kinase (1638 U/L; reference range 10 to 200 U/L), and lactate dehydrogenase (1930 U/L; reference range 0 to 133 U/L). An incidental finding of anaplasmosis was made using a commercial ELISA assay kit (SNAP® 4DX®; IDEXX Laboratories, Inc., USA) in dog 1 and a whole blood specimen was submitted for polymerase chain reaction (PCR) analysis. Survey radiographs depicted tubular structures of soft tissue opacity between the colon and the urinary bladder in dog 1. Minimally displaced maxillary fractures, fissure lines between the lung lobes, and decreased abdominal serosal detail were noted in dog 2. On abdominal ultrasonography, generalized uterine enlargement and right ovarian cysts were identified in dog 1, and a markedly enlarged right uterine horn containing echogenic debris was discovered incidentally in dog 2. A tentative diagnosis of pyometra with anaplasmosis and pyometra with minimally displaced maxillary fractures were made in dog 1 and dog 2, respectively.
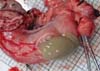
Dog 1 was treated for anaplasmosis and pyometra with doxycycline (5 mg/kg per os (PO) twice a day (BID); Doxycycline Hyclate Cap, Kukje Pharm Co., Ltd, Korea), amoxicillin-clavulanic acid (12.5 mg/kg PO BID; Amocla® Tab, Kuhnil Pharm Co., Ltd, Korea), metronidazole (15 mg/kg PO BID; Flasinyl Tab, CJ HealthCare Corp, Korea), enrofloxacin (5 mg/kg PO BID; Baytril® Flavour Tab, Bayer Animal Health GmbH, Germany), cephalexin (30 mg/kg PO BID; Cefaxin Cap, Hankook Korus Pharm Co., Ltd, Korea), and famotidine (0.5 mg/kg PO BID; Famotidine, Nelson Pharm Co., Ltd, Korea). Three days after initiating the medications, a high neutrophil count was still identified on Gram-stained vaginal smears, and a complete blood count revealed marked leukocytosis (46.63×103 cells/µl; reference range 6 to 17×103 cells/µl). Surgical intervention was elected for the management of pyometra. The dog was premedicated with atropine sulfate (0.04 mg/kg s.c; Atropine sulfate inj®, Je Il Pharm Co., Ltd, Korea). Anesthesia was induced with propofol (6 mg/kg i.v.; Provive 1%®, Myungmoon Pharm Co., Ltd, Korea) and maintained with isoflurane (Isoflurane®; Choongwae Co., Ltd, Korea) in oxygen. Normal saline (0.9%) was administered i.v. at a rate of 5 mL/kg/h during the surgical procedure. Cefazolin (30 mg/kg i.v.; Safdin®, Daehan Newpharm Co., Ltd, Korea) was administered at the time of anesthetic induction. Surgical exploration of the abdominal cavity was performed with the dog positioned in dorsal recumbency. A distended uterine body and horns were identified and ovariohysterectomy was performed. The uterine horns were found to be filled with purulent material (Fig. 1), and fluid from both horns was submitted separately for aerobic and anaerobic bacterial culture. Doxycycline (5 mg/kg PO BID), metronidazole (15 mg/kg i.v. BID), enrofloxacin (5 mg/kg s.c. BID), cefazolin (30 mg/kg i.v. three times a day (TID)), and famotidine (0.5 mg/kg i.v. BID) were administered postoperatively. The white blood cell count decreased within normal limits (13.96×103 cells/µl) two days post operation. All cultures were plated on sheep blood agar and both the aerobic and anaerobic cultures yielded no growth. A PCR assay was positive for Anaplasma phagocytophilum, the owner refused further treatment for this infection.
In dog 2, surgical correction of the maxillary fractures was deemed unnecessary since they were not displaced. Conservative management consisting of antibiotic therapy with cephradine (25 mg/kg i.v. BID; Panzedin inj, Hankook Korus Pharm Co., Ltd, Korea), supplemental oxygen, pain relief, and cage rest was instituted and the dog was monitored continuously. After a week of stabilization, surgical exploration of the abdominal cavity was performed. A distended right uterine horn was identified and ovariohysterectomy was performed. The uterine horns were found to be filled with purulent material as in a dog 1, and fluid from both horns was submitted separately for aerobic and anaerobic bacterial culture. Cephradine (25 mg/kg i.v. BID) and famotidine (0.5 mg/kg i.v. BID) were administered postoperatively. The white blood cell count revealed normal limits (16.81×103 cells/µl; reference range 6 to 17×103 cells/µl) 4 days post operation. All cultures yielded no growth.
Pyometra is a uterine infection resulting from migration of bacteria from the vagina (6). The severity of clinical signs depends on whether the cervix is open enough to allow drainage of purulent fluid. Depression, lethargy, vomiting, septicemia, toxemia, and shock are generally identified in cases of closed-cervix pyometra (1). Septicemia and toxemia are life-threatening conditions unless the source of the pathogens is promptly removed and appropriate antibiotics are administered (67). Therefore, surgical ovariohysterectomy followed by bacterial culture and antimicrobial susceptibility testing is considered the most effective treatment.
Bacteria including Escherichia coli (the predominant pathogen), Streptococcus species, Klebsiella species, Staphylococcus species, Pasteurella species, Proteus species, and Pseudomonas species are most often isolated from the uteri of bitches with pyometra (2). In this report, we share two cases with negative bacterial cultures despite the presence of purulent material within the lumen of the uterus. The etiology of a diagnosis of sterile pyometra despite the presence of purulent material is unknown; proposed explanations include the elimination of bacteria by host defense mechanisms, the method used to collect samples for bacteriological examination, and the eradication of bacteria by the empirical use of antibiotics preoperatively (278).
The reproductive tract possesses a variety of defense mechanisms which operate in concert to prevent infection or alter the course of an active infection (910). These defenses include passive factors, such as pH, mucus, and the epithelial barrier, and active factors, such as the inflammatory cascade and the secretion of humoral factors including mucin, acids, lysozymes, lactoferrin, peroxidase enzymes, antimicrobial proteins, and interferon-α (1011). Humoral immunity and cellular defense strategies may also be involved in rapid protection before antigenic stimulation (810). If these initial lines fail, a third strategy, which is acquired and antigen-specific, is employed that associates the humoral response with secretory IgA/IgM and locally produced IgG, as well as cellular immune responses (810). Additionally, the reproductive tract possesses several distinct features of the mucosal immune system (810). This includes the presence of endogenous flora, the predominance of IgG, hormonal fluctuations that may modify mucosal immunity, and the need to be tolerant of sperm and a developing fetus. The reproductive tract must also possess the ability to respond to sexually transmitted bacterial pathogens (910).
Presently available options for the collection of bacteriological samples include swabbing the walls of the uterine horns, harvesting uterine wall specimens, and obtaining samples of purulent material using sterile, disposable, syringes and needles (278). The absence of bacterial growth has been noted using each of the described techniques (78). In a study by Enginler et al. (2014), no bacterial growth was observed in 3 out of 15 uterine swab samples collected from a patient with pyometra (8). In a separate study (Hagman et al. 2006), no bacterial growth was detected in 1 out of 10 uterine wall sections measuring 1×1 cm obtained from a dog with pyometra (7). In the cases reported here, 3 mL of purulent material collected from both uterine horns was submitted for aerobic and anaerobic bacterial culture; no bacterial growth was identified in any sample. All three techniques presently available for the collection of bacteriological samples may yield no bacterial growth despite the presence of purulent material in some patients with pyometra.
If left untreated, pyometra can lead to death of the patient, since septicemia and toxemia can develop rapidly (2). Therefore, antimicrobial drugs are usually selected on the basis of experience and knowledge of resistance patterns for the predominant pathogen, Escherichia coli (2). The empirical use of preoperative antibiotics can reduce the bacterial burden and false-negative results might be obtained. In this study, we presume that the use of preoperative antibiotics in dog 1 had only a minor effect on bacterial numbers, since a high neutrophil count was still identified on Gram-stained vaginal smears and a complete blood count revealed marked leukocytosis in spite of treatment with antibiotics for three days. Therefore, negative bacterial cultures might not be the result of preoperative antibiotics.
A study including a large number of cases is warranted to better determine the overall rates of positive and negative cultures in three etiologic categories of sterile pyometra: the host defense mechanisms, the method used to collect samples for bacteriological examination, and the empirical use of preoperative antibiotics.
References
1. Pretzer SD. Clinical presentation of canine pyometra and mucometra: a review. Theriogenology. 2008; 70:359–363.

2. Agostinho JM, de Souza A, Schocken-Iturrino RP, Beraldo LG, Borges CA, Avila FA, Marin JM. Escherichia coli strains isolated from the uteri horn, mouth, and rectum of bitches suffering from pyometra: virulence factors, antimicrobial susceptibilities, and clonal relationships among strains. Int J Microbiol. 2014; 2014:979584.
3. Hagman R, Greko C. Antimicrobial resistance in Escherichia coli isolated from bitches with pyometra and from urine samples from other dogs. Vet Rec. 2005; 157:193–196.
4. Harada K, Niina A, Nakai Y, Kataoka Y, Takahashi T. Prevalence of antimicrobial resistance in relation to virulence genes and phylogenetic origins among urogenital Escherichia coli isolates from dogs and cats in Japan. Am J Vet Res. 2012; 73:409–417.

5. Soleymani Majd H, Watermeyer S, Ismail L. Pyometra presenting in conjunction with bowel cancer in a post-menopausal women: a case report. Cases J. 2008; 1:24.

6. Contri A, Gloria A, Carluccio A, Pantaleo S, Robbe D. Effectiveness of a modified administration protocol for the medical treatment of canine pyometra. Vet Res Commun. 2015; 39:1–5.

7. Hagman R, Kindahl H, Lagerstedt AS. Pyometra in bitches induces elevated plasma endotoxin and prostaglandin F2alpha metabolite levels. Acta Vet Scand. 2006; 47:55–67.
8. Enginler SO, Ates A, Diren Sıgırcı B, Sontas BH, Sonmez K, Karacam E, Ekici H, Evkuran Dal G, Gurel A. Measurement of C-reactive protein and prostaglandin F2alpha metabolite concentrations in differentiation of canine pyometra and cystic endometrial hyperplasia/mucometra. Reprod Domest Anim. 2014; 49:641–647.

9. Sundburg CR, Belanger JM, Bannasch DL, Famula TR, Oberbauer AM. Gonadectomy effects on the risk of immune disorders in the dog: a retrospective study. BMC Vet Res. 2016; 12:278.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download