Abstract
Foxp3 is a transcript factor for regulatory T cell development. Interestingly, Foxp3-expressing cells were identified in B cells, especially in CD19(+)CD5(+) B cells, while those were not examined in CD19(+)CD5(-) B cells. Foxp3-expressing CD5(+) B cells in this study were identified in human PBMCs and were found to consist of 8.5±3.5% of CD19(+)CD5(+) B cells. CD19(+)CD5(+)Foxp3(+) B cells showed spontaneous apoptosis. Rare CD19(+)CD5(+) Foxp3(+) regulatory B cell (Breg) population was unveiled in human peripheral blood mononuclear cells and suggested as possible regulatory B cells (Breg) as regulatory T cells (Treg). The immunologic and the clinical relevant of Breg needs to be further investigated.
CD5(+) B cells constitute a subset of B cells and a subpopulation of CD5(+) B cells produced IL-10 and suggested as a regulatory B cell, 'Br1' (1,2). The disease relationship of IL-10-producing regulatory B cells, especially in the human food allergy of atopic dermatitis, was described in a previous report of ours (3,4). Transcription factor Foxp3 is a transcript factor that controls the development of regulatory T cells and is involved in the negative regulation of immune responses (5). In this study, CD19(+)CD5(+)Foxp3(+) regulatory B cells (Breg) were demonstrated and characterized for the first time.
Venous blood was obtained from six normal subjects (mean age=15.3±3.5 years, M:F=3:3) who visited the Department of Allergy and Clinical Immunology at the Seoul Allergy Clinic for heath examination between March and June 2010; subjects had no specific disease. Signed consent forms were obtained from either the patient or the parent. The study was approved by the Institutional Review Board of Chungnam University Hospital, Daejeon, Korea.
Peripheral blood mononuclear cells (PBMCs) were isolated from venous blood using density-gradient separation and Ficoll-Hypaque (Biomedicals, Aurora, OH, USA). PBMCs were resuspended at 1×106 cells/ml in α-minimum essential medium (α-MEM; Irvine Scientific, Santa Ana, CA). CD19(+) CD5(+) Foxp3(+) regulatory B cells (Breg) were examined using fluorescence-labelled anti-CD5, anti-CD19, and anti-Foxp3 antibodies. Apoptotic characteristics were evaluated using fluorescence-labelled Annexin V.
The cells to be stained were prepared at a concentration of 1×106 cells in 100µl of FACS staining buffer (eBioscience) in an eppendorf tube. Cells were stained with specific monoclonal antibodies including allophycocyanin (APC)-labelled anti-CD19 (eBioscience) antibody, phycoerythrin-Cy7 (PE-Cy7)-labelled anti-CD5 antibody (eBioscience) and/or FITC-labelled Annexin V (eBioscience) for surface staining. at a concentration of 1µg/ml. After one hour incubation in the dark, cells were washed with FACS staining buffer three times. The cells were then resuspended in 100µl of permeabilisation/fixation buffer (ebioscience) just before intracellular staining. After finishing the cell surface staining and fixation/permeabilization, the cells were stained with a phycoerythrin (PE)-labelled anti-Foxp3 monoclonal antibody (eBioscience) in a dark room for 30 minutes at 4℃. After cell surface and intracellular staining, cells were resuspended in 500 ul of FACS staining buffer. The stained cells were acquired with a FACSCaliber (BD Biosciences, Milpitas, CA, USA), and the data were analysed with CellQuest software (BD Biosciences).
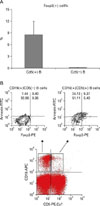
CD19(+) B cells expressed the intracellular Foxp3 transcript factor, especially only in a CD5(+) B cell subpopulation in B cells (Fig. 1). Foxp3 expression was exclusively identified in the CD5(+) population of CD19(+) B cells and Foxp3(+) cells fraction was 8.5±3.5% in CD19(+)CD5(+) B cells and 0.1±0.1 % in CD19(+)CD5(-) B cells (Fig. 2A). In the representative case, the apoptotic frequency of CD19(+)CD5(+) Foxp3(+) B cells was 63.44% (9.37%/(9.37+5.40%)) (Fig. 2B) and CD19(+)CD5(+) B cells showed highly apoptotic characteristics using Annexin V.
Forkhead/winged-helix protein, Foxp3, is a transcription factor that controls the development of regulatory T cells (Treg) and has been reported to be expressed only in CD4 (+) T cells and not in CD19(+) B cells or in CD8(+) T cells of mice (5). These CD4(+)Foxp3(+) T cells are well known as Treg; they participate in negative immune regulations (5). IL-10-producing CD5(+) B cells (Br1) also prevented and reversed allergic airway inflammation via Foxp3(+) T regulatory cells in a murine model (6). In this study, Foxp3-expressing CD5(+) B cells, as another subpopulation of CD5(+) B cell, were identified, in addition to IL-10-producing regulatory B cells (Br1) and TGF-β-producing regulatory B cells (Br3) (7). Moreover, CD19(+)CD5(+)Foxp3 (+) B cells showed high spontaneous apoptotic frequency. (Fig. 2B). In mice, 14% of B cells underwent spontaneous apoptosis (8). CD19(+)CD5 (+)Foxp3(+) B cells showed considerable spontaneous apoptosis as compared to other cell populations, especially to populations of B cells in human.
CD19(+)CD5(+)Foxp3(+) regulatory B cells (Breg) seemed to be another kind of regulatory B cells. It may be time to arrange systemically the nomenclature for regulatory B cell subsets; as IL-10-producing regulatory B cells as Br1 (4) like Tr1 of IL-10-producing T cells (5) and as TGF-β-producing regulatory B cells as Br3 (7) like Th3 of TGF-β-producing T cells (9). In addition, it has been suggested that CD19(+) CD5(+)Foxp3(+) B cells be defined as a Breg like Treg of T helper cells expressing transcript factor Foxp3.
Conclusively, CD19(+)CD5(+)Foxp3(+) regulatory B cells were identified for the first time in this study. CD19(+) CD5(+)Foxp3(+)B cells were suggested as possible regulatory B cells (Breg), a subpopulation of CD5(+) B1 cell, and were found to have highly apoptotic characteristics. The exact role and immunologic significance of CD19(+)CD5(+) Foxp3(+) regulatory B cells need to be further investigated.
Figures and Tables
Figure 1
Foxp3 expression in only CD5(+) cells in B cells. B cells were subgated by the surface CD19 expression and analyzed by the expression of CD5 and Foxp3.

Figure 2
Characteristics of Foxp3-expressing cells in CD19(+) B cells. (A) Foxp3(+) cell % in CD19(+)CD5(+) and CD19(+)CD5(-) B cell subpopulations. (B) Apoptotic characteristics of CD19(+)CD5(+) Foxp3(+) regulatory B cells (Breg) in a representative case. PBMCs were gated by the expression of CD19 and CD5. Each set of CD19 (+)CD5(-) B cells and CD19(+)CD5(+) B cells, CD19(-)CD5(-) cell was analyzed for Foxp3 expression and annexin V staining.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Chungbuk BIT Research-Oriented University Consortium).
References
2. Gieni RS, Umetsu DT, DeKruyff RH. Ly1- (CD5-) B cells produce interleukin (IL)-10. Cell Immunol. 1997. 175:164–170.
3. Lee JH, Noh J, Noh G, Kim HS, Mun SH, Choi WS, Cho S, Lee S. Allergen-specific B cell subset responses in cow's milk allergy of late eczematous reactions in atopic dermatitis. Cell Immunol. 2010. 262:44–51.

4. Noh J, Lee JH, Noh G, Bang SY, Kim HS, Choi WS, Cho S, Lee SS. Characterisation of allergen-specific responses of IL-10-producing regulatory B cells (Br1) in Cow Milk Allergy. Cell Immunol. 2010. 264:143–149.

5. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.

6. Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010. 125:1114–1124.

7. Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS. Allergen-specific TGF-β-producing CD19(+)CD5(+) Regulatory B-Cell (Br3) Responses in Human Late Eczematous Allergic Reactions to Cow's Milk. J Interferon Cytokine Res. 2011. in press.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download