Abstract
Biphenotypic acute leukemia (BAL) is a rare type of leukemia, comprises 4% of all acute leukemias. It is more common in adults and the clinical features, as related to marrow dysfunction, are similar to those found in other patients with acute leukemia. BAL commonly shows a dimorphic blast population with, one resembling lymphoblasts and the other resembling myeloblasts. The majority of BAL patients express B-lymphoid and myeloid markers. BAL can be diagnosed by morphologic studies and by a comprehensive panel of immunological markers, as well as cytogenetic/molecular studies, such as fluorescence in situ hybridization (FISH) and reverse transcriptase-polymerase chain reaction (RT-PCR). In addition, its prognosis is relatively poor. We present here a 27 year-old female patient who showed lymphoblasts and myeloblasts on her marrow studies and these cells were positive for myeloid and B-lymphoid markers on the immunophenotypic studies. Chromosome analysis revealed 46, XX, t(6;19)(p23;p13.1), t(9;22)(q34;q11.2). A major (b3a2) type of BCR-ABL1 mRNA transcript was detected by RT-PCR, and a 5'ABL1 deletion was identified by FISH.
References
1. Choi HW, Shin MG, Kim HJ, et al. Biphenotypic acute leukemia with BCR-ABL mRNA transcript b3a2 type: a case report with review of the literature. Korean J Lab Med. 2006; 26:249–54.
2. Shaffer LG, Slovak ML, Campbell LJ, eds. ISCN (2009) An International System for Human Cytogenetic Nomenclature. Basel: Karger. 2009.
3. Lee DS. Acute leukemia of ambiguous lineage. The Korean Society for Laboratory Medicine, editor. The Laboratory Medicine. 4th ed.Reston: E-Public;2009. p. 198–9.

4. Borowitz MJ, bene MC, Harris NL, Porwit A, Matutes E. Acute leukaemias of ambiguous lineage. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed.Lyon: IARC Press;2006. p. 150–2.
5. Legrand O, Perrot JY, Simonin G, et al. Adult biphenotypic acute leukaemia: an entity with poor prognosis which is related to unfavourable cytogenetics and P-glycoprotein over-expression. Br J Haematol. 1998; 100:147–55.

6. Cuneo A, Ferrant A, Michaux JL, et al. Philadelphia chromosome-positive acute myeloid leukemia: cy-toimmunologic and cytogenetic features. Haematologica. 1996; 81:423–7.
7. Montgomery KD, Winter SS, Frost JD, et al. Myeloid antigen positive acute lymphoblastic leukemia with the philadelphia translocation and a jumping translocation of 1q in a child. Leukemia. 2004; 18:1548–50.

8. Shin S, Park SS, Cho HI, Hur MN, Lee YJ. Analysis of breakpoints of bcr-abl fusion gene using RT-PCR. Korean J Lab Med. 1999; 19:369–74.
9. Park TS, Lee ST, Song JW, et al. Biphenotypic acute leukemia with b2a2 fusion transcript and trisomy 21. Cancer Genet and Cytogenet. 2009; 188:129–31.

10. Kim YK, Lee JJ, Lee BH, et al. Successful autologous peripheral blood stem cell transplantation after molecular remission using imatinib mesalate in a patient with philadelphia chromosome-positive acute myeloid leukemia. Korean J Hemato Stem Cell Trans. 2003; 8:55–8.
11. Morel F, KA C, Le Bris MJ, et al. Deletion of 5'ABL region in philadelphia chromosome-positive chronic myeloid leukemia: frequency, origin and prognosis. Leuk Lymphoma. 2003; 44:1333–8.
Fig. 1.
Leukemic cell morphology, Immunohistochemistry and special stain. (A) Leukemic blasts are variable in size and morphology with small lymphoid like blasts and large blasts having irregular nuclei, prominent nucleoli and abundant cytoplasm from bone marrow aspirate smear (H&E stain, ×1,000). (B) Blasts showing positive staining with anti-CD 10 monoclonal antibody (IHC stain, ×1,000). (C) Blasts showing positive staining with anti-CD 79a mon-oclonal antibody (IHC stain, ×1,000).
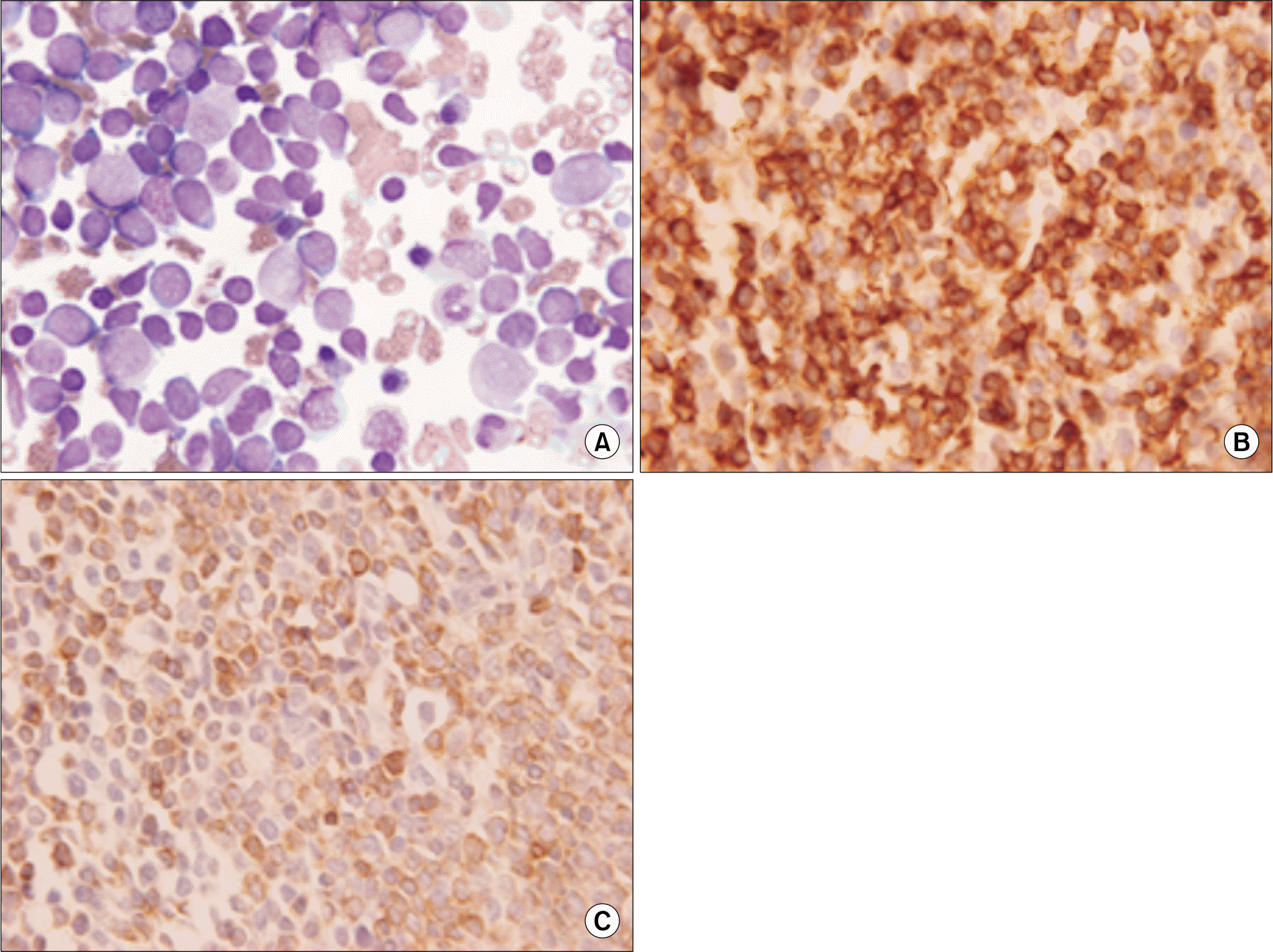
Fig. 2.
The representative karyogram reveals 46, XX, t(6;19) (p23;p13.1), t(9;22)(q34;q11.2)[7] (GTL banding, ×1,000).
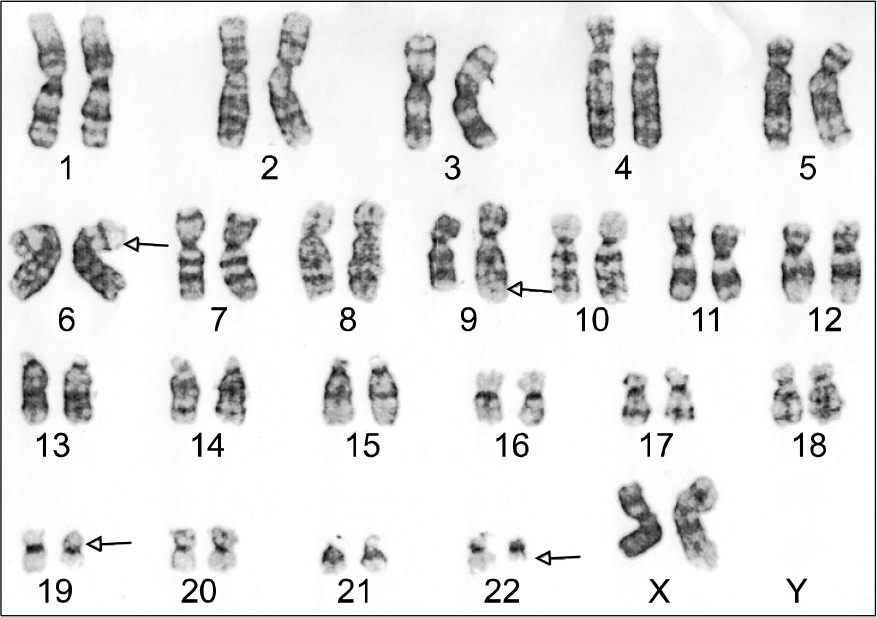
Fig. 3.
RT-PCR analysis for BCR-ABL1 chimeric mRNA discloses b3a2 type (B). positive control with b3a2 type fusion transcript (C) and size marker (A).
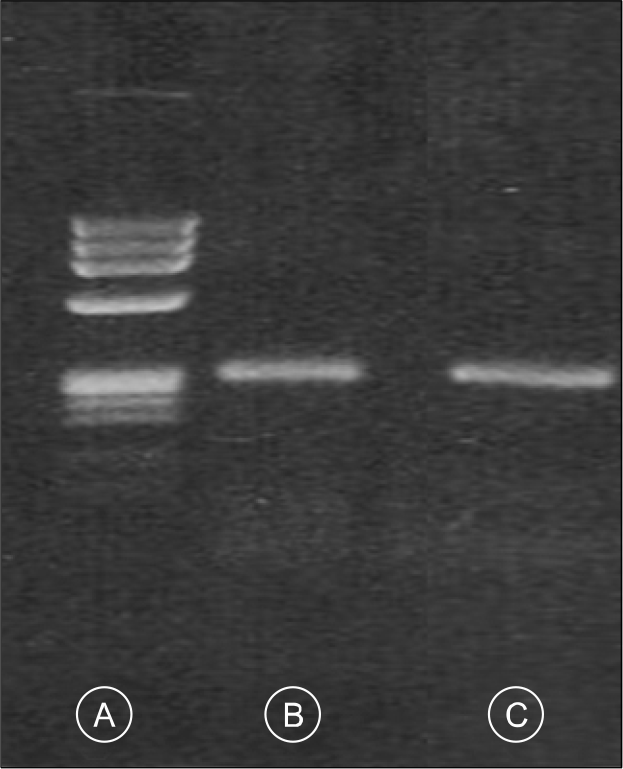
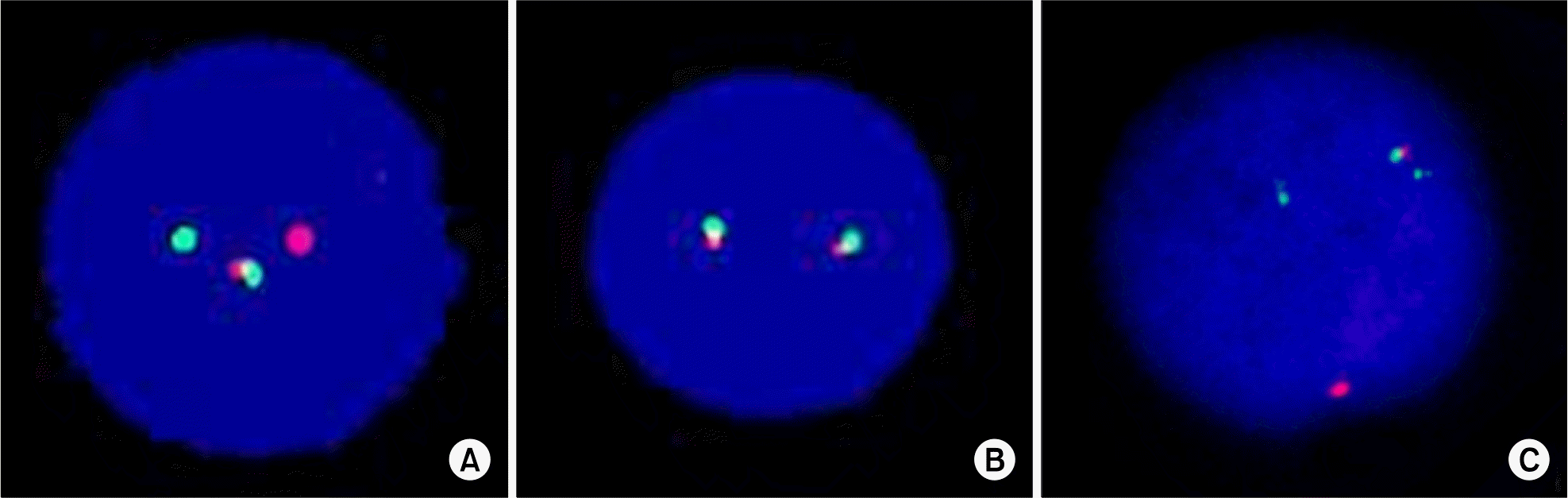
Fig. 4.
Interphase fluorescence in situ hybrideization (FISH) results. (A) FISH using LSI BCR-ABL1 ES Dual color Tanslocation Probe, reveals one green (native BCR), one large orange (native ABL1) and one fused orange-green (5'BCR-3'ABL1 fusion on der(22)t(9;22)) signal pattern. No smaller orange signal indicates the deletion of 5'ABL1 region on der(9)t(9;22). (B) FISH with TCF3 BAR probe shows two fusion signals indicating no rearrangements of TCF3 gene. (C) FISH using LSI BCR-ABL1 Dual color Dual Fusion Tanslocation Probe, reveals two green (native BCR and 3'BCR on der(9)t(9;22)), one orange (native ABL1) and one fused orange-green (5'BCR-3'ABL1 fusion on der(22)t(9;22)) signal pattern. No one orange signal indicates the deletion of 5'ABL1 on der(9)t(9;22).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download