Abstract
Multiple myeloma is a malignant disease of plasma cells, whereas ankylosing spondylitis is a chronic inflammatory disease of axial joints. The relationship between the two diseases is uncertain, but chronic inflammation could trigger multiple myeloma. The authors report the cases of two ankylosing spondylitis patients with a disease duration of more than 20 years, that subsequently developed IgA kappa and IgG lambda chain myeloma, respectively, and discuss the possible pathogenetic relationship between these diseases.
References
2. Rajkumar SV. MGUS and smoldering multiple myeloma: update on pathogenesis, natural history, and management. Hematology Am Soc Hematol Educ Program. 2006. 340–5.

3. Rajkumar SV, Lacy MQ, Kyle RA. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Blood Rev. 2007; 21:255–65.

4. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992; 6:225–47.

5. Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 2008; 111:3388–94.

6. Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008; 18:3–10.

7. Cowling P, Ebringer R, Ebringer A. Association of inflammation with raised serum IgA in ankylosing spondylitis. Ann Rheum Dis. 1980; 39:545–9.

8. O'Neill TW, Harrison BJ, Yin AL, Holt PJ. Ankylosing spondylitis associated with IgA lambda chain myeloma. Br J Rheumatol. 1997; 36:401–2.
9. Lam SM, Ho HH, Dunn P, Luo SF. Association of ankylosing spondylitis with IgA-multiple myeloma: report of a case and pathogenetic considerations. Taiwan Yi Xue Hui Za Zhi. 1989; 88:726–8.
11. Kim YN, Lee HE, Lee SH, et al. Ankylosing spondylitis associated with plasmacytoma: a case report. J Korean Rheum Assoc. 2005; 12:240–4.
12. Renier G, Renier JC, Gardembas-Pain M, Chevailler A, Boasson M, Hurez D. Ankylosing spondylitis and monoclonal gammopathies. Ann Rheum Dis. 1992; 51:951–4.

13. Blanc AP, Gastaut JA, Sebahoun G, Carcassonne Y. Association for ankylosing spondylarthritis multiple myeloma. A case history (author's transl). Sem Hop. 1979; 55:1335–7.
14. Gualandi M, Trotta F, Faggioli M, Vanini A, Tassinari MC. Association of non-secreting myeloma and ankylosing spondylitis. Considerations on a clinical case. Minerva Med. 1981; 72:2631–7.
Fig. 1.
(A) Serum protein electrophoresis (Left) showed a sharp peak in the gamma globulin region. The gamma globulin region composed 37.5% of total (8.92 g/dL). Immunofixation electrophoresis (IFE, Right) showed a mono-clonal IgA, κ band. (B) Serum protein electrophoresis (Left) showed a prominent M-peak (39.9% of total protein, 8.78 g/ dL), whereas immunofixation electrophoresis (IFE, Right) showed a monoclonal IgG, λ band.
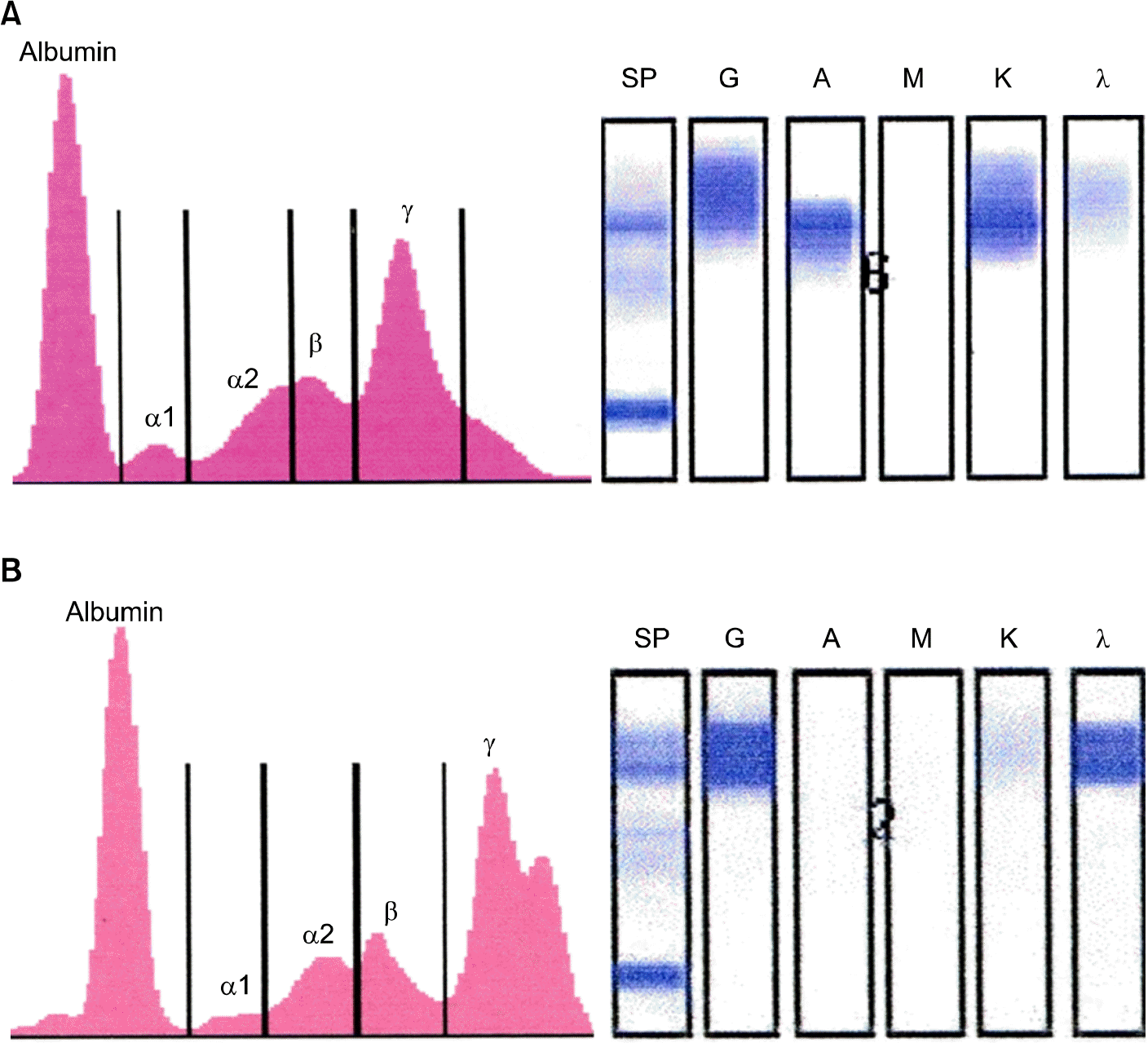
Fig. 2.
(A) Skull radiograph showing multiple variably sized punched-out lesions in the fronto-parietal area. (B) D-L spine AP LAT radiograph showing extensive ankylosis and syndesmophytes in the thoracic and upper lumbar spine.
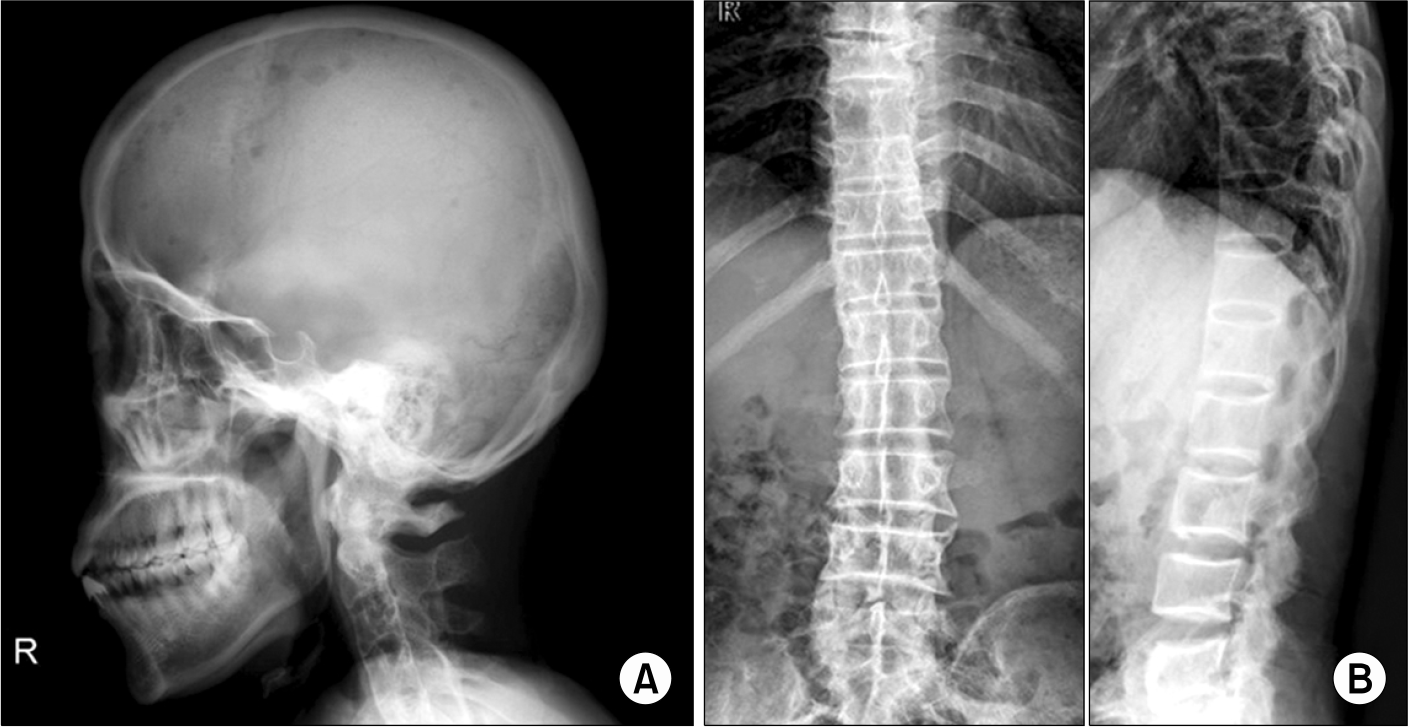
Fig. 3.
PET scan showing multifocal increased uptakes in vertebrae, pelvic bones, clavicles, rib cages, and both humeri and femurs. No changes in uptake were observed after dexamethasone treatment.
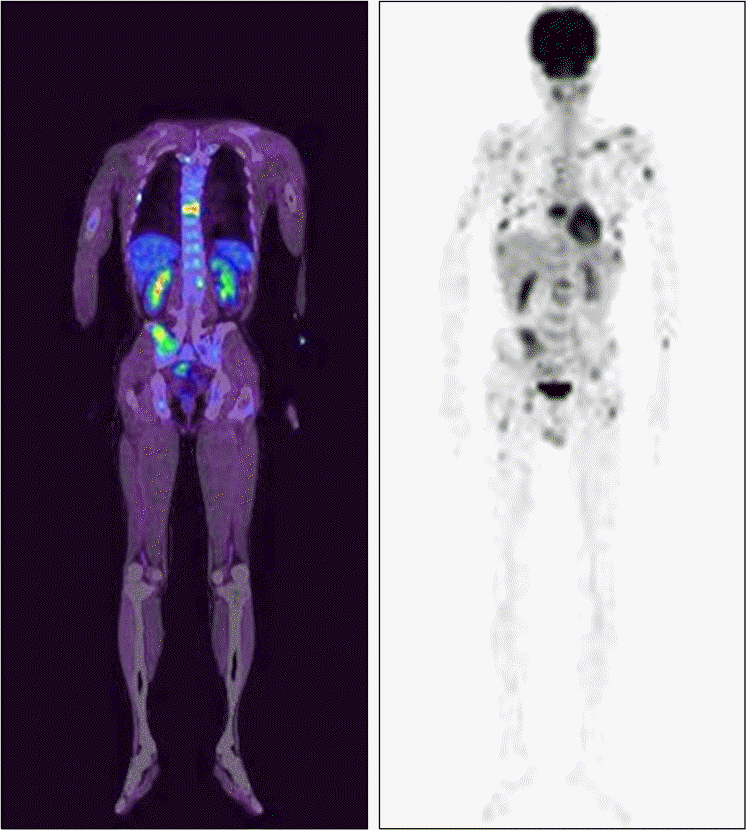
Table 1.
A summary of data from previous reports concerning the association between ankylosing spondylitis and plasma cell disorders




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download