Abstract
Background:
The ability of non-myeloablative allogeneic stem cell transplants to eradicate host neoplastic cells is based on the accumulating evidence of a graft-versus-malignancy (GVM) effect. Stable mixed chimerism (MC) is associated to the lower risk for the development of graft-versus-host diseases (GVHD), but this possibly occurs at the expense of the GVM effect. Therefore, assessment of the chimerism status is critical to allow immune intervention to maintain a state of donor-host tolerance and to prevent loss of the graft.
Methods:
Serial post-transplant peripheral blood samples were collected from 17 patients with various malignant diseases following non-myeloablative allogeneic stem cell transplantation. DNA was amplified from the T-cells, and the polymerase chain reaction (PCR) products were quantified by an automated fluorescent DNA analyzer.
Results:
All 17 patients showed T-cell MC at post-transplant, but this varied in degree and duration, and then 3 patterns emerged. Group 1: 5 patients experienced a short interval of T-cell MC prior to conversion to complete donor chimerism (CC) (median: 25 days). Group 2: 5 patients showed a rapid increase of host cells after a brief MC at a median of 21 days. They never achieved CC, and they relapsed or showed progressive diseases. Group 3: 7 patients showed persistent T-cell MC for 40-50 days, and they subsequently gradually converted to CC after a median of 112 days.
Conclusion:
All the patients achieved T-cell MC in post-transplant, but the CC development differed in frequency and speed. GVHD preceded the onset of T-cell CC in the majority of the patients. Serial engraftment monitoring of the T-cell chimerism status during the first 100 days after non-myeloablative stem cell transplantation is important in aiding the clinical management of such patients.
REFERENCES
1). Petersen SL., Madsen HO., Ryder LP, et al. Haematopoietic stem cell transplantation with non-myeloablative conditioning in the outpatient setting: results, complications and admission requirements in a single institution. Br J Haematol. 2004. 125:225–31.

2). Cao TM., Shizuru JA., Wong RM, et al. Engraftment and survival following reduced-intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood. 2005. 105:2300–6.

3). Mielcarek M., Storb R. Graft-vs-host disease after non-myeloablative hematopoietic cell transplantation. Leuk Lymphoma. 2005. 46:1251–60.

4). Ruiz-Arguelles GJ., Gomez-Almaguer D., David-Gomez-Rangel J, et al. Allogeneic hematopoietic stem cell transplantation with non-myeloablative conditioning in patients with acute myelogenous leukemia eligible for conventional allografting: a prospective study. Leuk Lymphoma. 2004. 45:1191–5.
5). Arya M., Gommersall LM., Shergill IS., Patel HR., Chao D. Non-myeloablative allogeneic stem cell transplantation: a promising new therapy in the management of metastatic renal cell cancer. BJU Int. 2005. 96:474–5.

6). Rzepecki P., Zolnierek J., Sarosiek T., Langiewicz P., Szczylik C. Allogeneic non-myeloablative hematopoietic stem cell transplantation for treatment of metastatic renal cell carcinoma-single center experience. Neoplasma. 2005. 52:238–42.
7). Bornhauser M., Thiede C. Chimerism analysis after allogeneic stem cell transplantation. Haematologica. 2005. 90:1301A.
8). Seidel MG., Fritsch G., Matthes-Martin S, et al. In vitro and in vivo T-cell depletion with myeloablative or reduced-intensity conditioning in pediatric hematopoietic stem cell transplantation. Haematologica. 2005. 90:1405–14.
9). Van Deerlin VM., Leonard DG. Bone marrow engraftment analysis after allogeneic bone marrow transplantation. Clin Lab Med. 2000. 20:197–225.

10). Nuckols JD., Rasheed BK., McGlennen RC., Bigner SH., Stenzel TT. Evaluation of an automated technique for assessment of marrow engraftment after allogeneic bone marrow transplantation using a commercially available kit. Am J Clin Pathol. 2000. 113:135–40.

11). Thiede C., Bornhauser M., Ehninger G. Evaluation of STR informativity for chimerism testing - comparative analysis of 27 STR systems in 203 matched related donor recipient pairs. Leukemia. 2004. 18:248–54.

12). Fredriksson M., Barbany G., Liljedahl U., Hermanson M., Kataja M., Syvanen AC. Assessing hematopoietic chimerism after allogeneic stem cell transplantation by multiplexed SNP genotyping using microarrays and quantitative analysis of SNP alleles. Leukemia. 2004. 18:255–66.

13). Huh HJ., Huh JW., Suk Eun, et al. Clinical significance of mixed chimerism after hematopoietic stem cell transplantation. Korean J Lab Med. 2002. 22:441–6.
14). Kim TY., Park SH., Kwon EH., Kim KY., Suh JS., Sohn SK. The discrimination power and effectiveness of 3 kinds of LTR primers in the VNTR-PCR for evaluation of the engraftment of allogeneic peripheral blood stem cells transplantation. Korean J Clin Pathol. 2001. 21:527–33.
15). Promega PowerPlex 16 System. Technical Manual No. D012. 2001. URL:. http://www.promega.com/tbs/tmd012/tmd012.pdf.
16). Lion T. Summary: reports on quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection. Leukemia. 2003. 17:252–4.

17). Wasch R., Bertz H., Kunzmann R., Finke J. Incidence of mixed chimaerism and clinical outcome in 101 patients after myeloablative conditioning regimens and allogeneic stem cell transplantation. Br J Hae-matol. 2000. 109:743–50.
18). Roman J., Serrano J., Jimenez A, et al. Myeloid mixed chimerism is associated with relapse in bcr-abl positive patients after unmanipulated allogeneic bone marrow transplantation for chronic myelogenous leukemia. Haematologica. 2000. 85:173–80.
19). Bader P., Beck J., Frey A, et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant. 1998. 21:487–95.

20). de Weger RA., Tilanus MG., Scheidel KC., van den Tweel JG., Verdonck LF. Monitoring of residual disease and guided donor leukocyte infusion after allogeneic bone marrow transplantation by chimaerism analysis with short tandem repeats. Br J Hae-matol. 2000. 110:647–53.
21). Choi SJ., Lee KH., Lee JH, et al. Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant. 2000. 26:327–32.

22). Bertheas MF., Lafage M., Levy P, et al. Influence of mixed chimerism on the results of allogeneic bone marrow transplantation for leukemia. Blood. 1991. 78:3103–6.

23). Najfeld V., Burnett W., Vlachos A., Scigliano E., Isola L., Fruchtman S. Interphase FISH analysis of sex-mismatched BMT utilizing dual color XY probes. Bone Marrow Transplant. 1997. 19:829–34.

24). Dubovsky J., Daxberger H., Fritsch G, et al. Kinetics of chimerism during the early post-transplant period in pediatric patients with malignant and non-malignant hematologic disorders: implications for timely detection of engraftment, graft failure and rejection. Leukemia. 1999. 13:2059–69.

25). McCarthy NJ., Bishop MR. Nonmyeloablative allogeneic stem cell transplantation. Oncologist. 2000. 5:487–96.
Fig. 1
Representative engraftment analysis results from the case No. 2 patient. The loci shown is penta E. Allele numbers and peak areas are designated in the boxes below each allele peak. (A) Donor. (B) Pre-transplantation patient. (C) Mixed chimerism at post-transplantation day 7. (D) Complete donor chimerism at post-transplantation day 28.
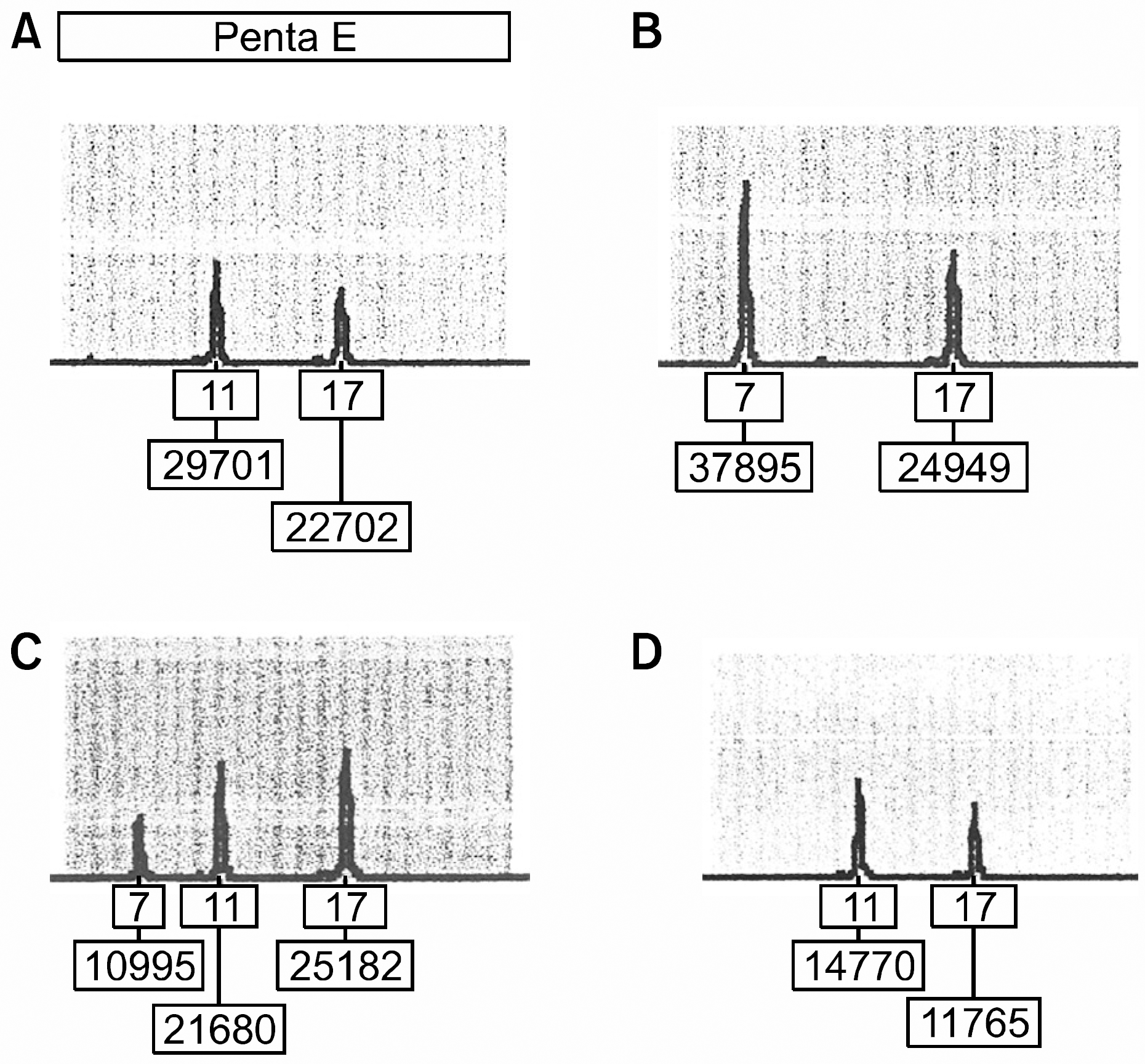
Fig. 2
Chimerism analysis result. (A) Case No. 2 patient showed a rapid conversion to CC. (B) Case No. 13 patient showed a brief interval of T-cell MC followed by a rapid loss of graft and disease relapse despite of DLI. (C) Case No. 4 patient showed a persistent T-cell MC at day 40~50 (>15%) and later gradually converted to CC. Abbreviations: GVHD, graft-versus-host diseases; CC, complete chimerism; MC, mixed chimerism; DLI, donor lymphocyte infusion.
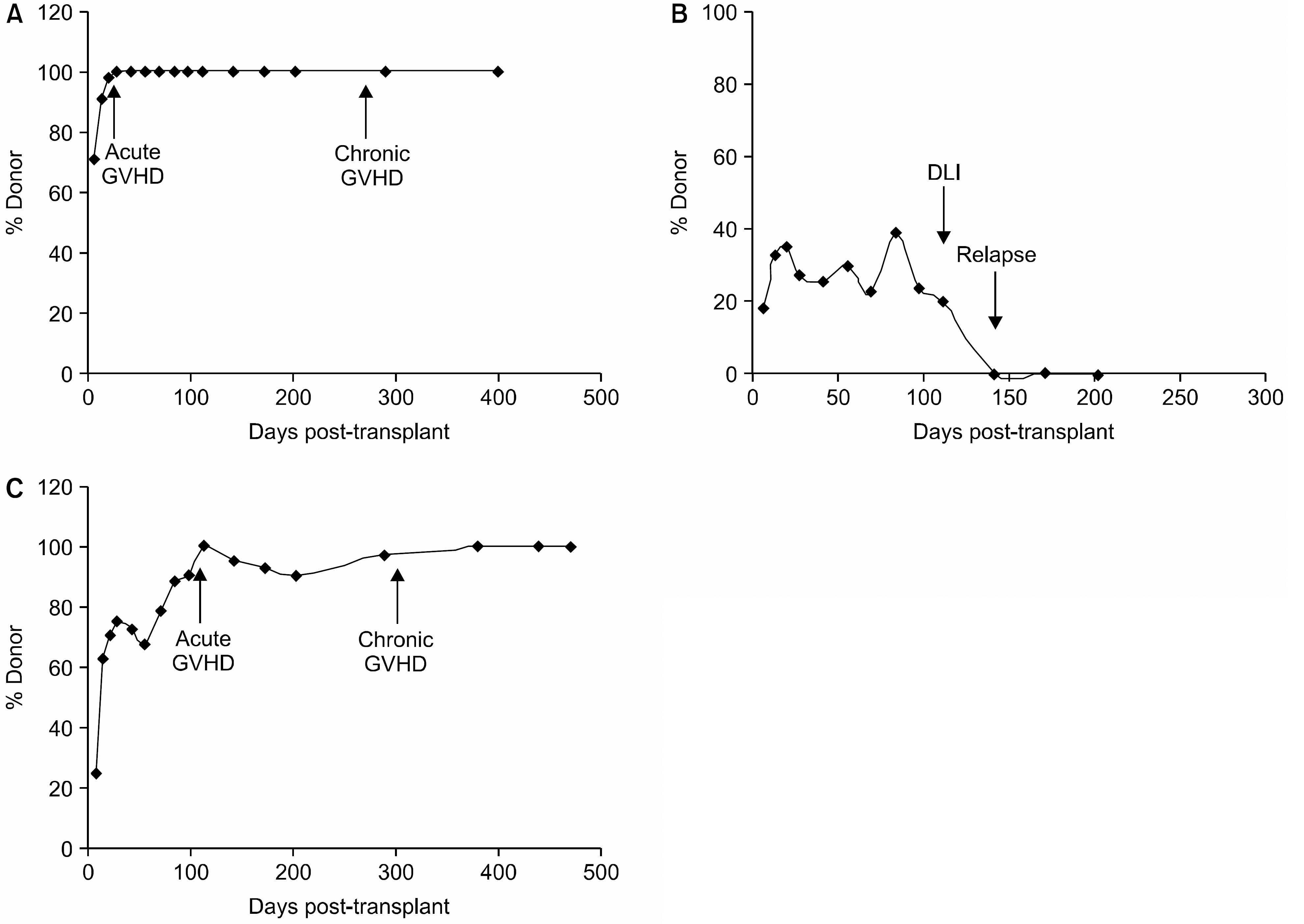
Table 1.
The PowerPlex16 System locus-specific informations
Abbreviations: STR, short tandem repeats; TH1, throsine hydroxylase theta l; FGA, alpha chain fibrinogen; TPOX, thyroid peroxidase; vWA, von willebrand factor; CSF1PO, c-fms-proto-oncogene for CSF-1 receptor; TMR, carboxy-tetramethylrhodamine; FL, fluorescein; JOE, 6-carboxy-4', 5'- dichloro-2', 7'-dimethoxyfluorescein; NA, not applicable.
Table 2.
Clinical and hematologic findings in 17 patients
Table 3.
Follow-up informations




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download