Abstract
Background:
The response rates and survival following allogeneic bone marrow transplantation (BMT) or immunosuppressive treatment were compared in severe aplastic anemia (SAA) and the prognostic factors related with survival identified.
Methods:
Medical data of SAA patients, treated with BMT or immunosuppressive therapy (IST) at the Ajou University Hospital, between January 1995 and December 2005, were retrospectively analyzed.
Results:
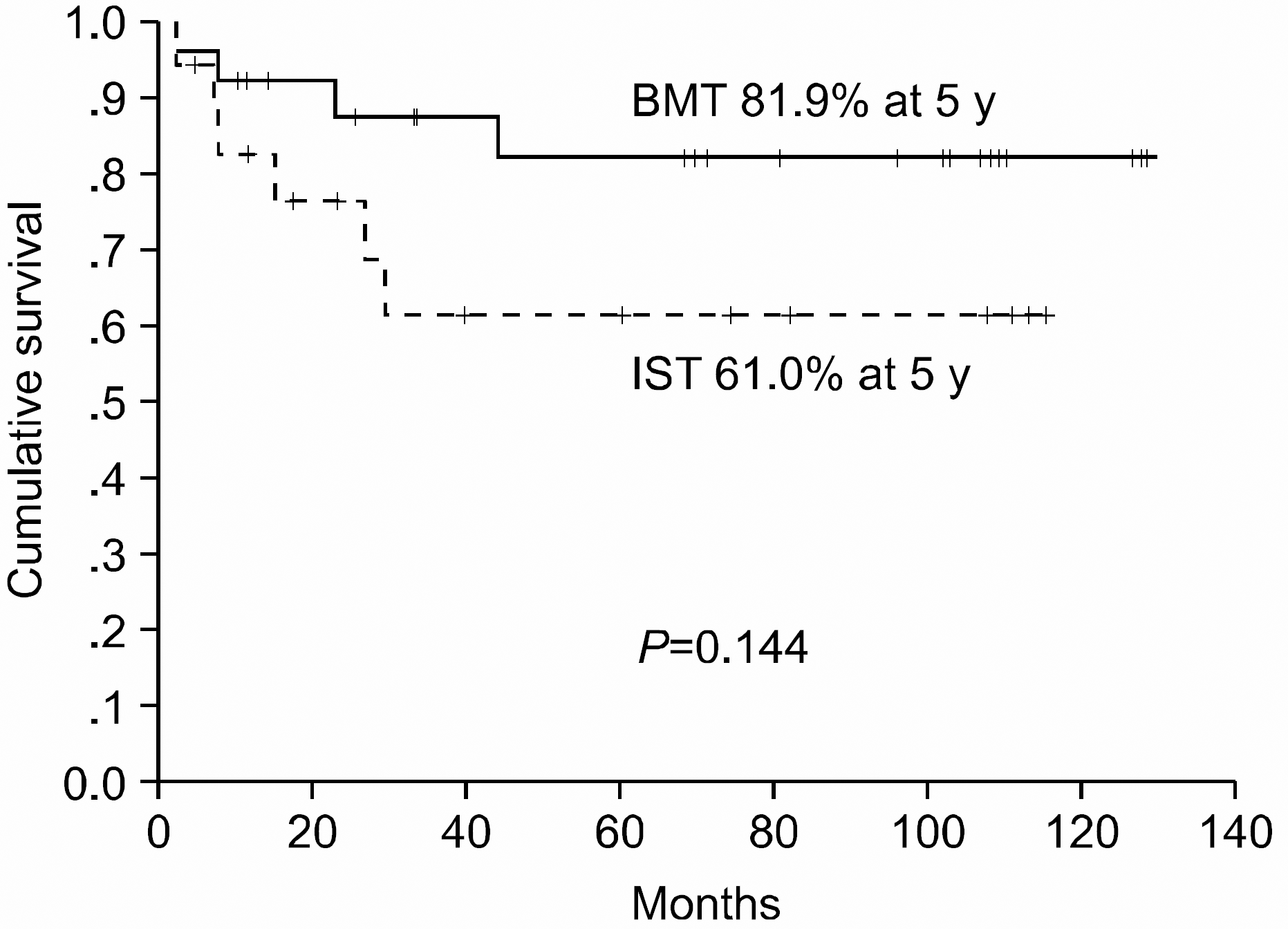
A total of 43 patients were evaluable; 18 (41.9%) were treated with IST (antithymocyte globulin plus cyclosporine A plus steroid) and 25 (58.1%) with allogeneic BMT. In the IST group, the response rate was 77.8% (2 complete and 12 partial remissions), with two treatment failures. As later complications, acute myeloid leukemia developed in 1 patient and myelodysplastic syndrome developed in 2. In the BMT group, the response rate was 92.0% (18 complete and 5 partial remissions) (P<0.001). Six patients developed grade II to III acute graft-versus-host-disease (GVHD) and 3 developed chronic GVHD. The median survival time in all patients was 60.27 months, and the 5-year survival rates were 61.0 and 81.9% in the IST and BMT groups, respectively (P=0.144). The factors influencing the overall survival were an age under 40-years and a positive treatment response.
Conclusion:
This study shows that allogeneic BMT, compared to IST, resulted in good response andoverall survival rates in patients with SAA. However, the overall survival rate between the two groups was statistically insignificant. Our study suggests that younger age SAA patients, with HLA-matched BMT donors, may benefit more from allogeneic BMT.
REFERENCES
1). Gale RP., Champlin RE., Feig SA., Fitchen JH. Aplastic anemia: biology and treatment. Ann Intern Med. 1981. 95:477–94.

2). Young NS. Aplastic anemia, myelodysplasia, and related bone marrow failure syndromes. Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 16th ed.United States of America: McGraw-Hill Companies;2005. p. 617–26.
4). Bagby GC., Lipton JM., Sloand EM., Schiffer CA. Marrow failure. Hematology Am Soc Hematol Educ Program. 2004. 318–36.

5). Maciejewski JP., Risitano AM. Aplastic anemia: management of adult patients. Hematology Am Soc Hematol Educ Program. 2005. 110–7.

6). Kim I., Yoon SS., Park S., Kim BK., Kim NK. The treatment of severe aplastic anemia: outcomes of bone marrow transplantation and immunosuppressive therapy in a single institution of Korea. J Korean Med Sci. 2003. 18:365–71.

7). Sohn HJ., Jang GD., Shin YR, et al. Survival according to treatment modalities in 137 patients with aplastic anemia. Korean J Hematol. 2003. 38:1–7.
8). Ahn MJ., Choi JH., Lee YY, et al. Outcome of adult severe or very severe aplastic anemia treated with immunosuppressive therapy compared with bone marrow transplantation: multicenter trial. Int J Hematol. 2003. 78:133–8.

10). Frickhofen N., Rosenfeld SJ. Immunosuppressive treatment of aplastic anaemia with antithymocyte globulin and cyclosporine. Semin Hematol. 2000. 37:56–68.
11). Mehta J., Singhal S. Graft failure: diagnosis and management. Barrett J, Treleaven J, editors. The clinical practice of stem-cell transplantation. Oxford, England: Isis Medical Media;1998. p. 645–58.
12). Camitta BM., Storb R., Thomas ED. Aplastic anemia (first of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982. 306:645–52.
14). Marsh J., Schrezenmeier H., Marin P, et al. Prospective randomized multicenter study comparing cyclosporin alone versus the combination of antithymocyte globulin and cyclosporin for treatment of patients with nonsevere aplastic anemia: a report from the European Blood and Marrow Transplant (EBMT) Severe Aplastic Anaemia Working Party. Blood. 1999. 93:2191–5.

15). Gluckman E., Esperou-Bourdeau H., Baruchel A, et al. Multicenter randomized study comparing cyclosporine-A alone and antithymocyte globulin with prednisone for treatment of severe aplastic anemia. Blood. 1992. 79:2540–6.

16). Frickhofen N., Heimpel H., Kaltwasser JP., Schrezenmeier H. German Aplastic Anemia Group. Antithymocyte globulin with or without cyclosporin A; 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003. 101:1236–42.

17). Rosenfeld S., Follmann D., Nunez O., Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003. 289:1130–5.
18). Bacigalupo A., Brand R., Oneto R, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy. The European Group for Blood and Marrow Transplantation experience. Semin Hematol. 2000. 37:69–80.
19). Socie G., Henry-Amar M., Bacigalupo A, et al. Malignant tumors occurring after treatment of aplastic anemia, for The European Bone Marrow Transplan-tation–Severe Aplastic Anemia Working Party. N Engl J Med. 1993. 329:1152–7.
20). Ades L., Mary JY., Robin M, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004. 103:2490–7.

21). Horowitz MM. Current status of allogeneic bone marrow transplantation in acquired aplastic anemia. Semin Hematol. 2000. 37:30–42.

22). Margolis DA., Casper JT. Alternative-donor hematopoietic stem-cell transplantation for severe aplastic anemia. Semin Hematol. 2000. 37:43–55.

Table 1.
Patient characteristics
Table 2.
Treatment outcome
Table 3.
Univariate analysis of factors influencing overall survival
Table 4.
Multivariate analysis of factors influencing overall survival




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download