Abstract
Introduction
This study evaluated the capability of silk fibroin (SF) and recombinant human bone morphogenetic protein-2 loaded SF (SF-BMP) as a bone defect replacement matrix when grafted in a calvarial bone defect of rats in vivo.
Materials and Methods
A total 70 calvarial critical size defects (5.0 mm in diameter) made on 35 adult female Sprague-Dawley rats were used in this study. The defects were transplanted with (1) rhBMP-2 loaded silk fibroin graft (SF-BMP: 0.8+10 μ g), (2) Silk fibroin (SF: 10 μ g), and (3) no graft material (Raw). The samples were evaluated with soft x-rays, alkaline phosphatase activity, calcium/phosphate quantification, histological and histomorphometric analysis at postoperative 4 and 8 weeks.
Results
The SF-BMP group (48.86±14.92%) had a significantly higher mean percentage bone area than the SF group (24.96±11.01%) at postoperative 4 weeks.(P<0.05) In addition, the SF-BMP group (40.01±12.43%) had a higher % bone area at postoperative 8 weeks than the SF group (33.26±5.15%). The mean ratio of gray scale levels to the host bone showed that the SF-BMP group (0.67±0.08) had a higher mean ratio level than the SF group (0.61±0.09) at postoperative 8 weeks. These differences were not statistically significant.(P=0.168 and P=0.243, respectively) The ratio of the calcium and phosphate contents of the SF-BMP (0.93±0.22) group was lower than that of the SF (1.90±1.42) group at postoperative 4 weeks. However, the SF-BMP group (0.75±0.31) had a higher Ca/PO4 ratio than the SF (0.68±0.04) at postoperative 8 weeks. These differences were not statistically significant.(P=0.126 and P=0.627, respectively) For the bone-specific alkaline phosphatase (ALP) activity, which is recognized as a reliable indicator of the osteoblast function, the SF-BMP (23.71±8.60 U/L) groups had a significantly higher value than the SF group (12.65± 6.47 U/L) at postoperative 4 weeks.(P<0.05) At postoperative 8 weeks, the SF-BMP (21.65±10.02 U/L) group had a lower bone-specific ALP activity than the SF group (16.72±7.35 U/L). This difference was not statistically significant.(P=0.263) For the histological evaluation, the SF-BMP group revealed less inflammation, lower foreign body reactions and higher bone healing than the SF group at postoperative 4 and 8 weeks. The SF group revealed more foreign body reactions at postoperative 4 weeks. However, this immunogenic reaction decreased and the remnant of grafted material was observed at postoperative 8 weeks. For histomorphometric analysis, the SF-BMP group had a significantly longer bone length to total length ratio than those of the SF group at postoperative 4 and 8 weeks.(P<0.05)
References
2. Mizutani H, Urist MR. The nature of bone morphogenetic protein (BMP) fractions derived from bovine bone matrix gelatin. Clin Orthop Relat Res. 1982. 213–23.

3. Sato K, Urist MR. Bone morphogenetic protein-induced cartilage development in tissue culture. Clin Orthop Relat Res. 1984; 183:180–7.

4. Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988; 242:1528–34.

5. Sampath TK, Reddi AH. Homolgy of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci U S A. 1983; 80:6591–5.
6. Chai Y, Slavkin HC. Biology of bone induction and its clinical applications. Oral Maxillofac Surg Clin North Am. 1994; 7:739–53.

7. Wozney JM. Biology and clinical applications of rhBMP-2. Lynch SE, Genco RJ, Marx RE, editors. Tissue engineering: applications in maxillofacial surgery and periodontics. Chicago: Quintessence;1999. p. 103–10.
8. Komaki M, Katagiri T, Suda T. Bone morphogenetic protein-2 does not alter differentiation pathway of committed progenitors of osteoblasts and chondroblasts. Cell Tissue Res. 1996; 284:9–17.
9. Nam JH, Park JC, Yu SB, Chung YI, Tae GY, Kim JJ, et al. Bone regeneration with MMP sensitive hyaluronic acid-based hydrogel, rhBMP-2 and nanoparticles in rat calvarial critical size defect (CSD) model. J Korean Assoc Oral Maxillofac Surg. 2009; 35:137–45.
10. Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003; 55:1613–29.

11. Lee JH, Kim SM, Park JC, Sung MA, Yu SB, Nam JH, et al. Bone regeneration with hyaluronic acid based hydrogel-nanoparticle complex and rhBMP-2 in rat critical size defect model. Tissue Eng Regen Med. 2009; 6:730–8.
12. Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000; 65:389–402.

13. Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002; 59:573–84.
14. Chung YI, Ahn KM, Jeon SH, Lee SY, Lee JH, Tae G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J Control Release. 2007; 121:91–9.

15. Saito N, Okada T, Horiuchi H, Ota H, Takahashi J, Murakami N, et al. Local bone formation by injection of recombinant human bone morphogenetic protein-2 contained in polymer carriers. Bone. 2003; 32:381–6. s.

16. Boyan BD, Lohmann CH, Somers A, Niederauer GG, Wozney JM, Dean DD, et al. Potential of porous poly-D,L-lactide-co-glycolide particles as a carrier for recombinant human bone morphogenetic protein-2 during osteoinduction in vivo. J Biomed Mater Res. 1999; 46:51–9.
17. Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004; 25:5735–42.

18. Dal Pra I, Freddi G, Minic J, Chiarini A, Armato U. De novo engineering of reticular connective tissue in vivo by silk fibroin nonwoven materials. Biomaterials. 2005; 26:1987–99.

19. Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, et al. In vitro degradation of silk fibroin. Biomaterials. 2005; 26:3385–93.

20. Gosline JM, DeMont ME, Denny MW. The structure and properties of spider silk. Endeavour. 1986; 10:37–43.

21. Furuzono T, Ueki M, Kitamura H, Oka K, Imai E. Histological reaction of sintered nanohydroxyapatite-coated cuff and its fibroblast-like cell hybrid for an indwelling catheter. J Biomed Mater Res Part B Appl Biomater. 2009; 89:77–85.

22. Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006; 27:4434–42.

23. Kirker-Head C, Karageorgiou V, Hofmann S, Fajardo R, Betz O, Merkle HP, et al. BMP-silk composite matrices heal critically sized femoral defects. Bone. 2007; 41:247–55.

24. Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, et al. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J Biomed Mater Res A. 2006; 78:324–34.
25. Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, et al. Silk implants for the healing of critical size bone defects. Bone. 2005; 37:688–98.

26. Jang ES, Park JW, Kweon H, Lee KG, Kang SW, Baek DH, et al. Restoration of peri-implant defects in immediate implant installations by Choukroun platelet-rich-fibroin and silk fibroin powder combination graft. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:831–6.
27. Lee EH, Kim JY, Kweon HY, Jo YY, Min SK, Park YW, et al. A combination graft of low-molecular-weight silk fibroin with Choukroun platelet-rich fibrin for rabbit calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:e33–8.

28. Kim JY, Choi JY, Jeong JH, Jang ES, Kim AS, Kim SG, et al. Low molecular weight silk fibroin increases alkaline phosphatase and type I collagen expression in MG63 cells. BMB Rep. 2010; 43:52–6.

29. Minoura N, Tsukada M, Nagura M. Fine structure and oxygen permeability of silk fibroin membrane treated with methanol. Polymer. 1990; 31:265–9.

30. Santin M, Motta A, Freddi G, Cannas M. In vitro evaluation of the inflammatory potential of the silk fibroin. J Biomed Mater Res. 1999; 46:382–9.

Fig. 1.
Midsagittal reference line for histomorphometric analysis. A. Harvested calvaria, B. Soft X-ray image.
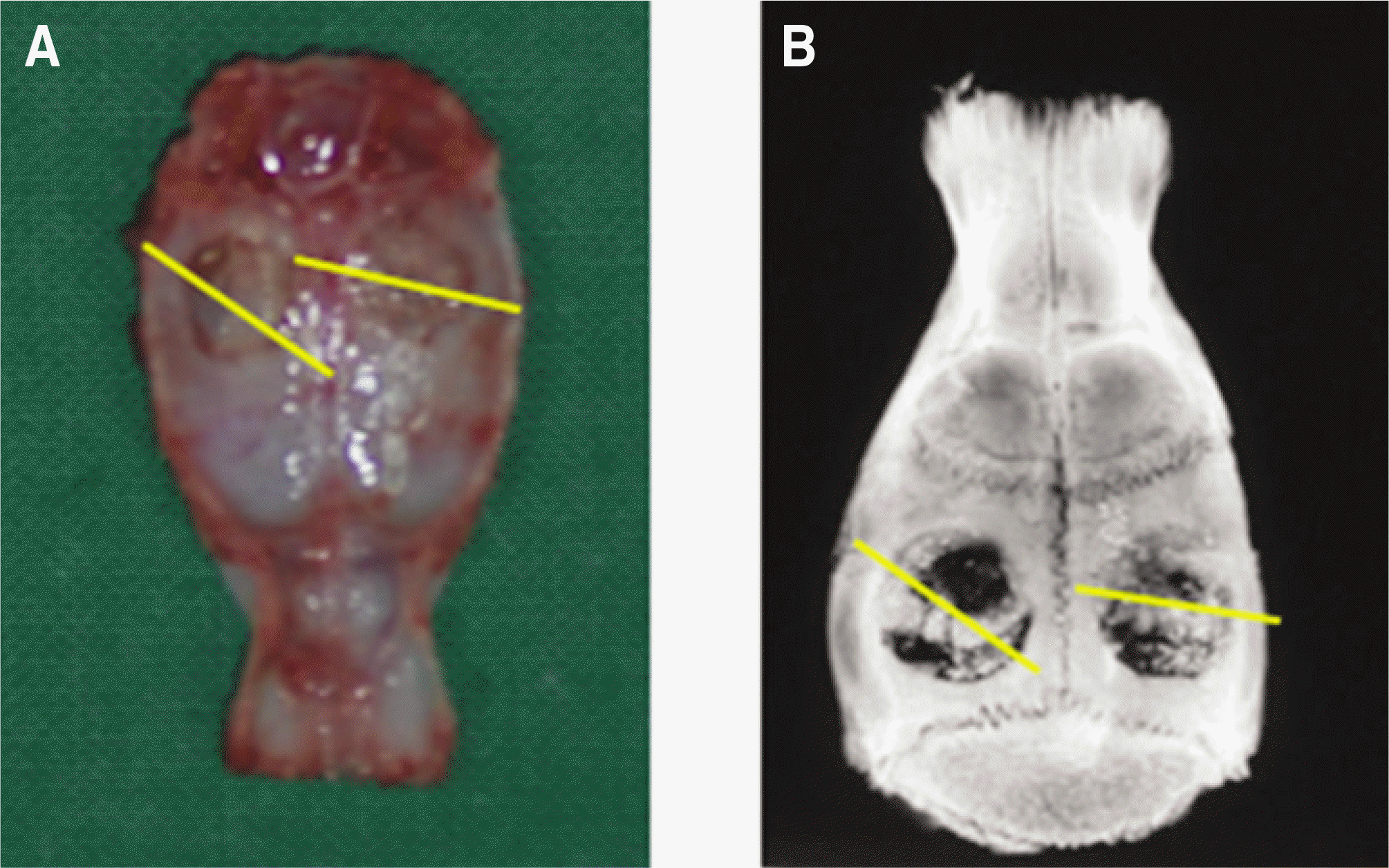
Fig. 2.
% bone area of densitometric analysis. A. The SF-BMP group (48.86±14.92%) had a significant higher value than that of SF group (24.96±11.01%, *P<0.05) at postoperative 4 weeks. B. The SF-BMP group (40.01±12.43%) had a higher %bone area than that of SF group (33.26±5.15%) at postoperative 8 weeks. This difference was not, however, statistically significant.(P=0.168) (SF: silk fibroin, SF-BMP: recombinant human bone morphogenetic protein-2 loaded SF)
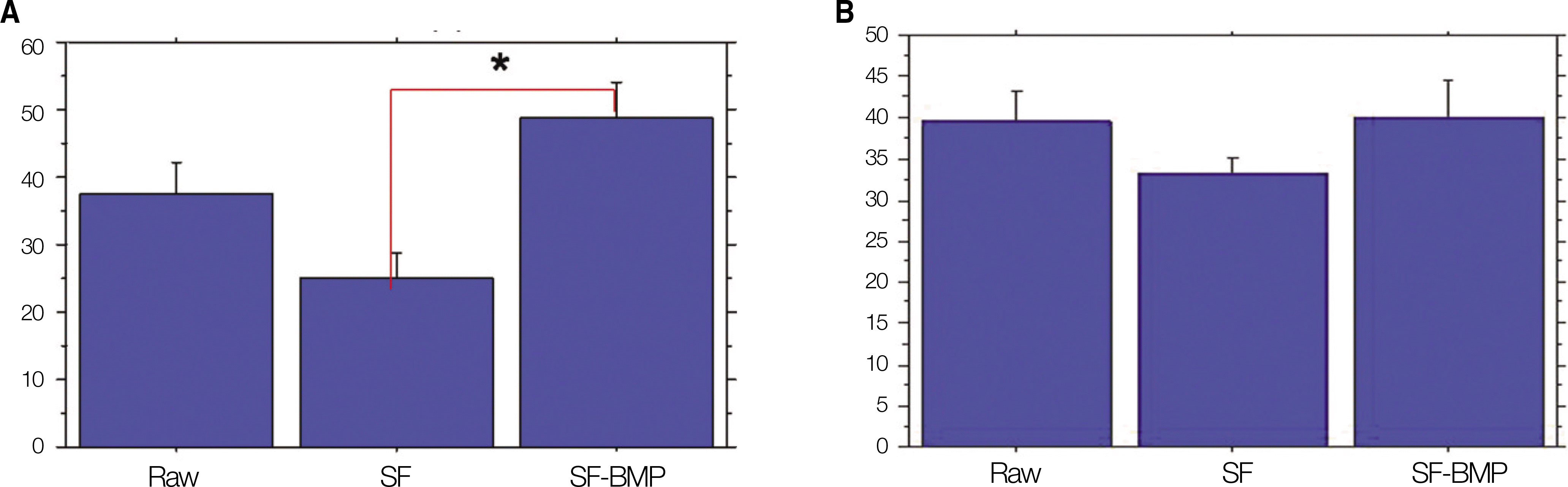
Fig. 3.
Gray scale ratio to host bone. A. The SF-BMP group (0.70±0.07) and SF group (0.70±0.09) had a higher value than that of the Raw group (0.67±0.12) at postoperative 4 weeks. These differences were not, however, statistically significant.(P=0.418 and P=0.424, respectively) B. At postoperative 8 weeks, the SF-BMP group (0.67±0.08) had a higher value than that of the SF group (0.61±0.09), however, it was not statistically significant.(P=0.243) (SF: silk fibroin, SF-BMP: recombinant human bone morphogenetic protein-2 loaded SF)
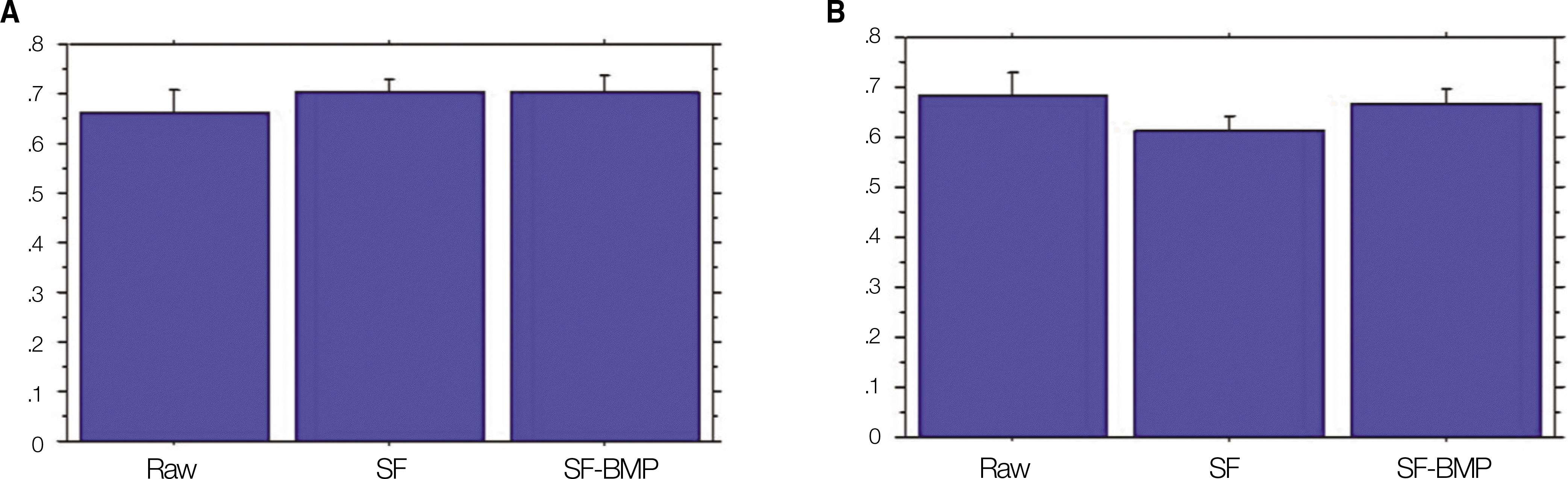
Fig. 4.
ALP activity. A. The SF-BMP group (23.71±8.60 U/L) had a significant higher value than that of the SF group (12.65± 6.47 U/L) at postoperative 4 weeks.(*P<0.05) B. The SF-BMP group (21.65±10.02 U/L) had a higher value than that of the SF group (16.72±7.35 U/L) at postoperative 8 weeks. However, there was not statistically significant difference.(P=0.263) (ALP: alkaline phosphatase, SF: silk fibroin, SF-BMP: recombinant human bone morphogenetic protein-2 loaded SF)
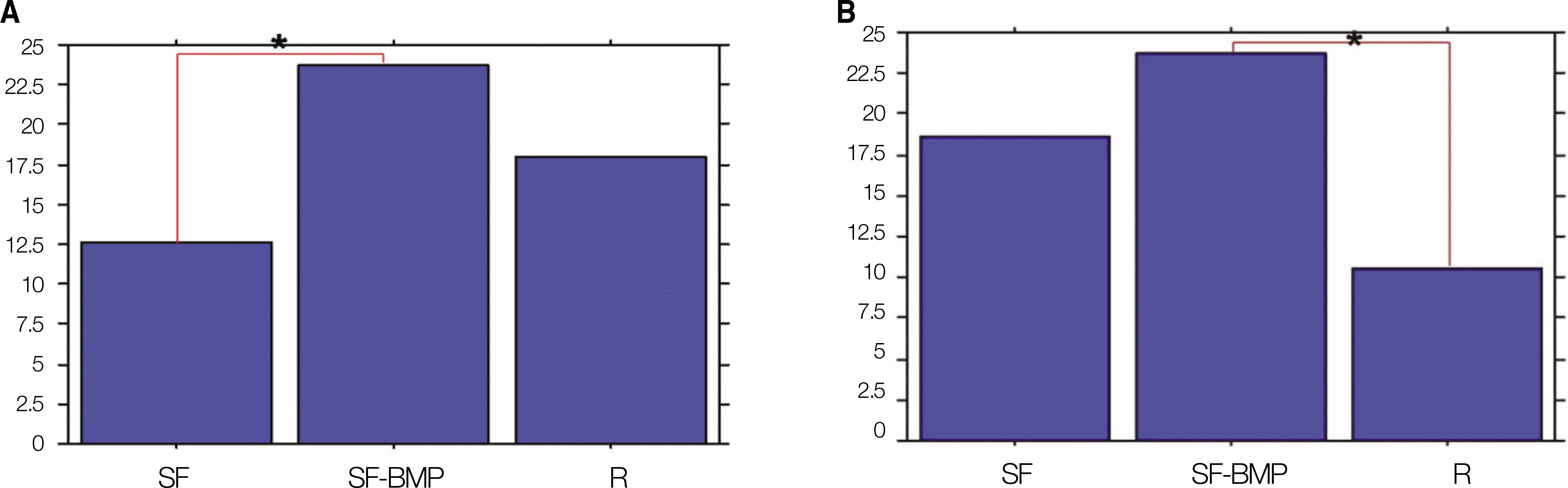
Fig. 5.
Ca2+ quantification. A. The SF-BMP group (28.67±14.28 mg/dL) had a significant higher value than that of the SF group (12.53±5.01 mg/dL) at postoperative 4 weeks.(*P<0.05) B. The SF-BMP group (28.56±20.44 mg/dL) had a significant higher value than that of the SF group (2.83±1.38 mg/dL) at postoperative 8 weeks.(*P<0.05) (SF: silk fibroin, SF-BMP: recombinant human bone morphogenetic protein-2 loaded SF)
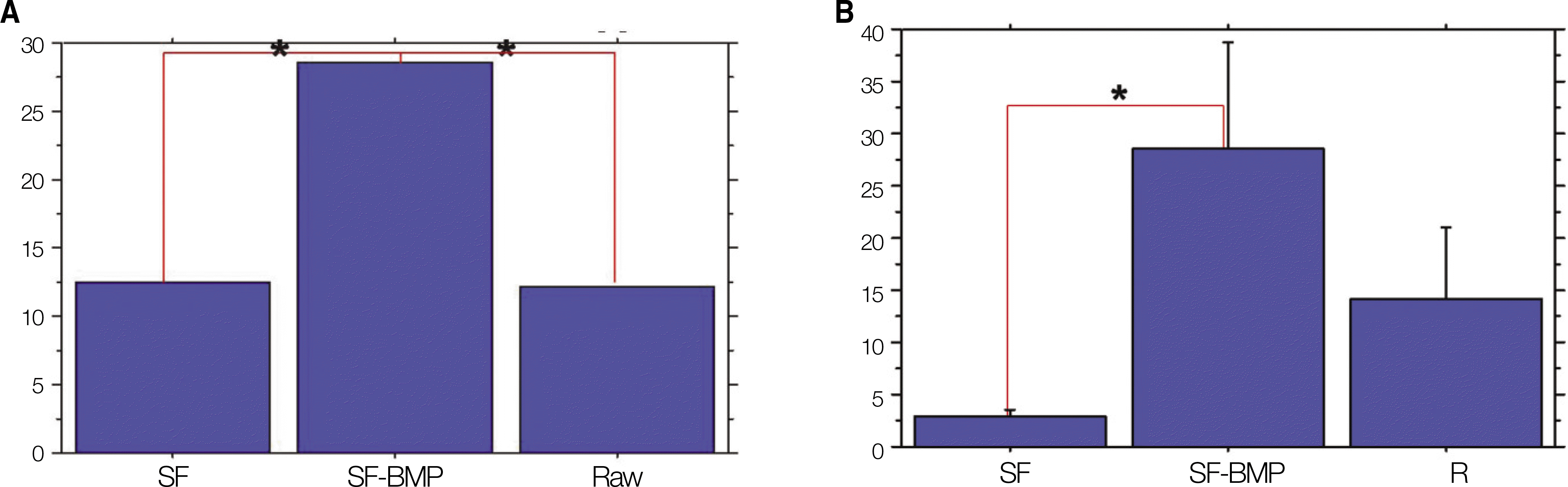
Fig. 6.
PO42- quantification. A. The SF-BMP group (30.77±12.52 mg/dL) had a significant higher value than that of the SF group (12.28±11.87 mg/dL) at postoperative 4 weeks.(*P<0.05) B. The SF-BMP group(34.09±12.37 mg/dL) had a significant higher value than that of the SF group (4.25±2.34 mg/dL)at postoperative 8 weeks.(*P<0.05) (SF: silk fibroin, SF-BMP: recombinant human bone morphogenetic protein-2 loaded SF)
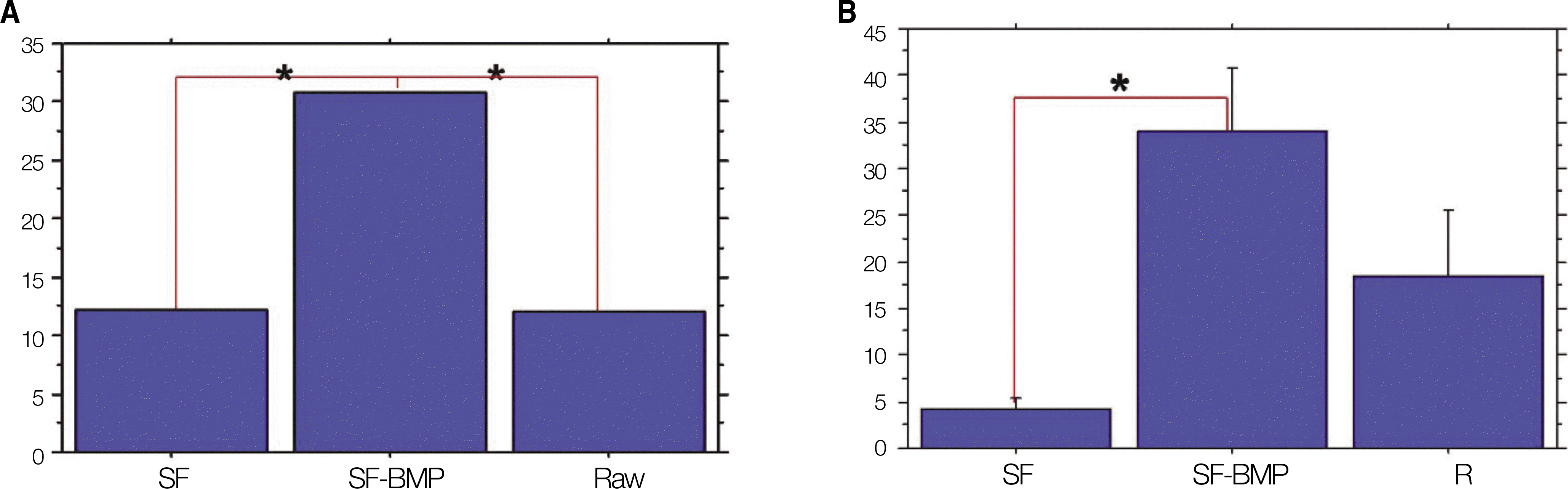
Fig. 7.
A. Postoperative 4 weeks.(H&E staining, original magnification x100) B. Postoperative 8 weeks.(H&E staining, original magnification x100) C. Postoperative 4 weeks.(Masson's trichrome staining, original magnification x100) D. Postoperative 8 weeks.(Masson's trichrome staining, original magnification x100) E. Postoperative 4 weeks.(Osteocalcin immunoreactive staining, original magnification x100) F. Postoperative 8 weeks.(Osteocalcin immunoreactive staining, original magnification x100)(rhBMP-2: recombinant human bone morphogenetic protein-2, SF: silk fibroin, SF-BMP: recombinant human bone morphogenetic protein-2 loaded SF)
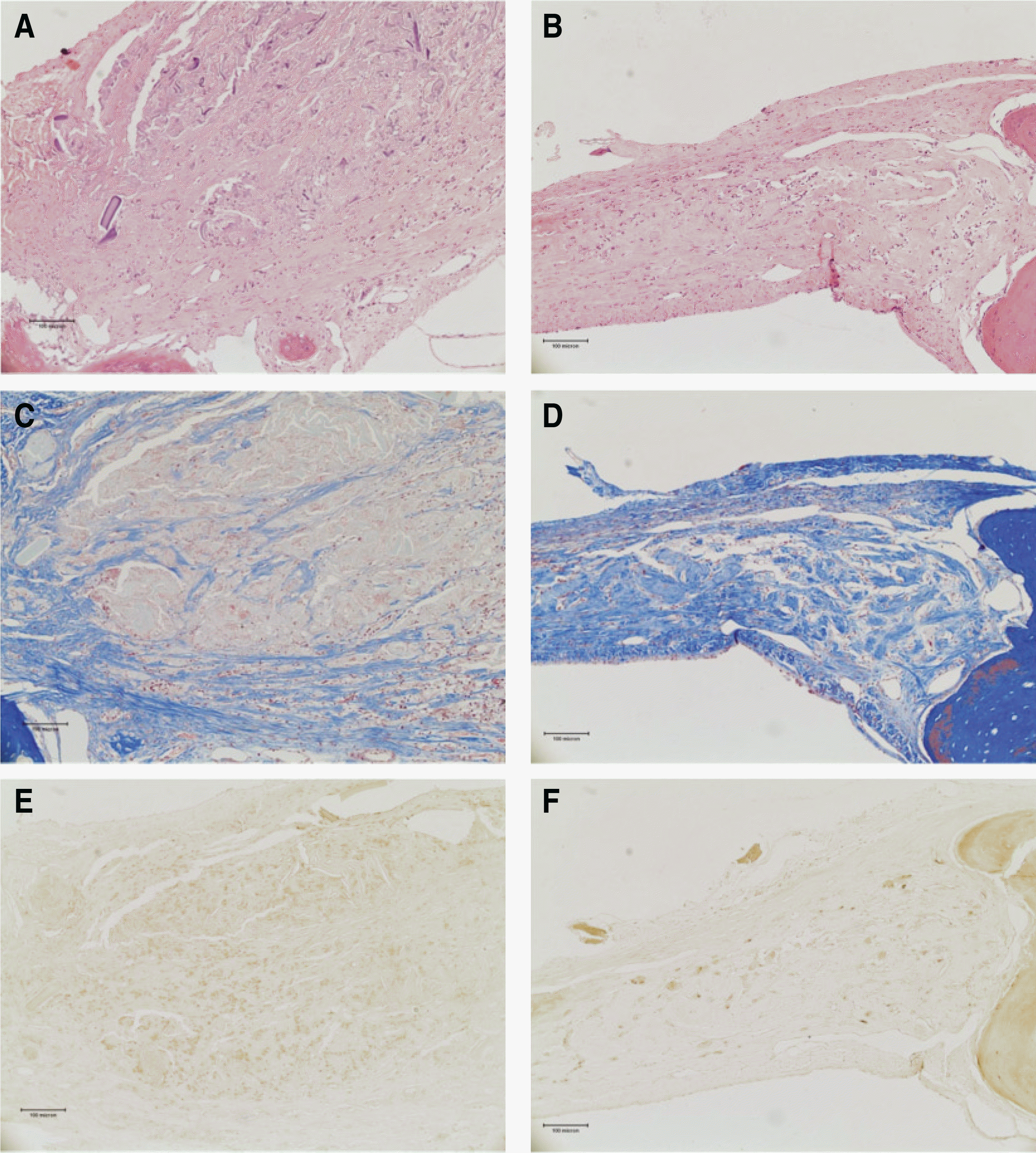
Fig. 8.
A. Postoperative 4 weeks.(H&E staining, original magnification x100) B. Postoperative 8 weeks.(H&E staining, original magnification x100) C. Postoperative 4 weeks.(Masson's trichrome staining, original magnification x100) D. Postoperative 8 weeks.(Masson's trichrome staining, original magnification x100) E. Postoperative 4 weeks.(Osteocalcin immunoreactive staining, original magnification x100) F. Postoperative 8 weeks.(Osteocalcin immunoreactive staining, original magnification x100)(rhBMP-2: recombinant human bone morphogenetic protein-2, SF: silk fibroin)

Table 1.
Animal grouping
| Groups | N (sites/animals) | Composition | Graft | |
|---|---|---|---|---|
| 4 weeks | 8 weeks | |||
| SF-BMP | 12 (6) | 10 (6) | rhBMP-2 loaded silk fibroin | 0.8+10 μ g |
| SF | 12 (6) | 12 (6) | Silk fibroin | 10 μ g |
| Raw | 12 (6) | 12 (5) | Raw defect | None |
Table 2.
Dry weight
| Groups | 4 weeks (mg) | 8 weeks (mg) |
|---|---|---|
| SF-BMP | 635.83±21.09 | 32.17±23.05 |
| SF | 611.75±4.03 | 7.00±2.83 |
| Raw | 616.83±13.35 | 28.17±13.47 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download