Abstract
Introduction
Skeletal homeostasis is normally maintained by the stability between bone formation by osteoblasts and bone resorption by osteoclasts. However, the correlation between the inflammatory reaction and osteoblastic differentiation of cultured osteoprogenitor cells has not been fully investigated. This study examined the effects of inflammatory cytokines on the osteoblastic differentiation of cultured human periosteal-derived cells.
Materials and Methods
Periosteal-derived cells were obtained from the mandibular periosteum and introduced into the cell culture. After passage 3, the periosteal-derived cells were further cultured in an osteogenic induction Dulbecco's modified Eagle's medium (DMEM) medium containing dexamethasone, ascorbic acid, and β-glycerophosphate. In this culture medium, tumor necrosis factor (TNF)-αwith different concentrations (0.1, 1, and 10 ng/mL) or interleukin (IL)-1β with different concentrations (0.01, 0.1, and 1 ng/mL) were added.
Results
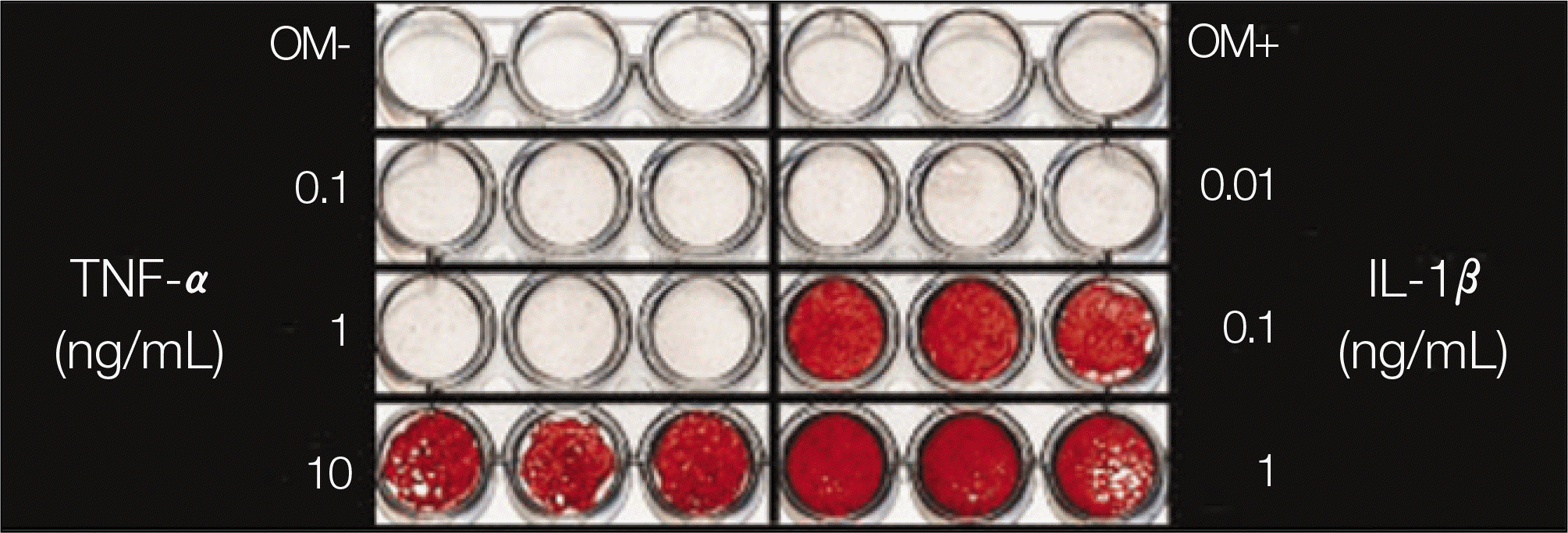
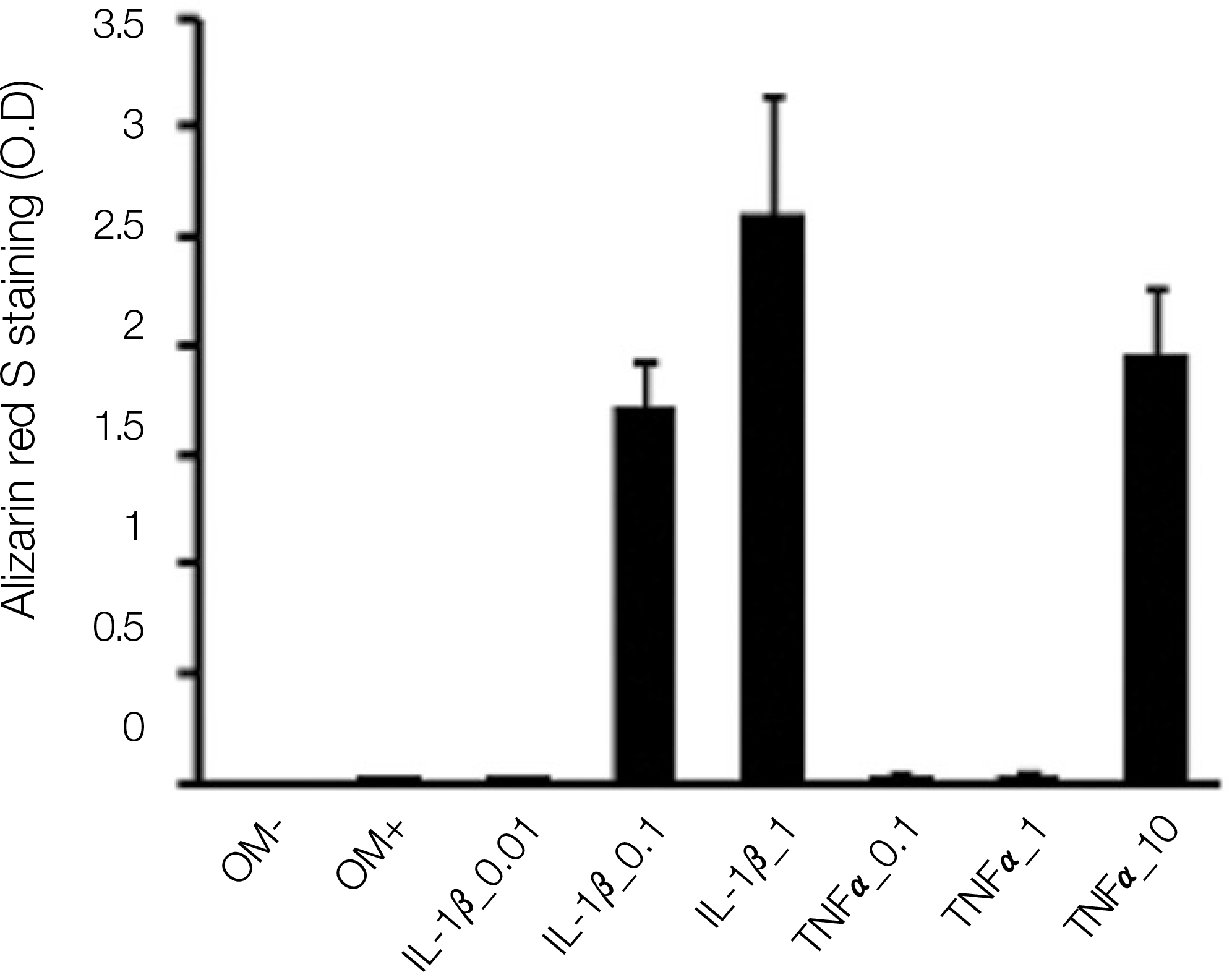
Both TNF-αand IL-1βstimulated alkaline phosphatase (ALP) expression in the periosteal-derived cells. TNF-αand IL-1βincreased the level of ALP expression in a dose-dependent manner. Both TNF-αand IL-1β also increased the level of alizarin red S staining in a dose-dependent manner during osteoblastic differentiation of cultured human periosteal-derived cells.
References
12. Park BW, Byun JH, Lee SG, Hah YS, Kim DR, Cho YC, et al. Evaluation of osteogenic activity and mineralization of cultured human periosteal-derived cells. J Korean Assoc Maxillofac Plast Reconstr Surg. 2006; 28:511–9.
13. Park BW, Byun JH, Ryu YM, Hah YS, Kim DR, Cho YC, et al. Correlation between vascular endothelial growth factor signaling and mineralization during osteoblastic differentiation of cultured human periosteal-derived cells. J Korean Assoc Maxillofac Plast Reconstr Surg. 2007; 29:197–205.
14. Walsh NC, Gravallese EM. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010; 233:301–12.

15. Li YP, Stashenko P. Proinflammatory cytokines tumor necrosis factoralpha and IL-6, but not IL-1, downregulate the osteocalcin gene promoter. J Immunol. 1992; 148:788–94.
16. Panagakos FS, Hinojosa LP, Kumar S. Formation and mineralization of extracellular matrix secreted by an immortal human osteoblastic cell line: modulation by tumor necrosis factoralpha. Inflammation. 1994; 18:267–84.

17. Nanes MS. Tumor necrosis factoralpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003; 321:1–15.
18. Segal B, Rhodus NL, Patel K. Tumor necrosis factor (TNF) inhibitor therapy for rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:778–87.

19. Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002; 196:77–85.
Fig. 1.
ALP expression in the periosteal-derived cells treated with TNF-αat 3 days of culture. A. No treatment of TNF-α. B. Treatment of 0.1 ng/mL TNF-α. C. Treatment of 1 ng/mL TNF-α. D. Treatment of 10 ng/mL TNF-α. (ALP: alkaline phosphatase, TNF: tumor necrosis factor)
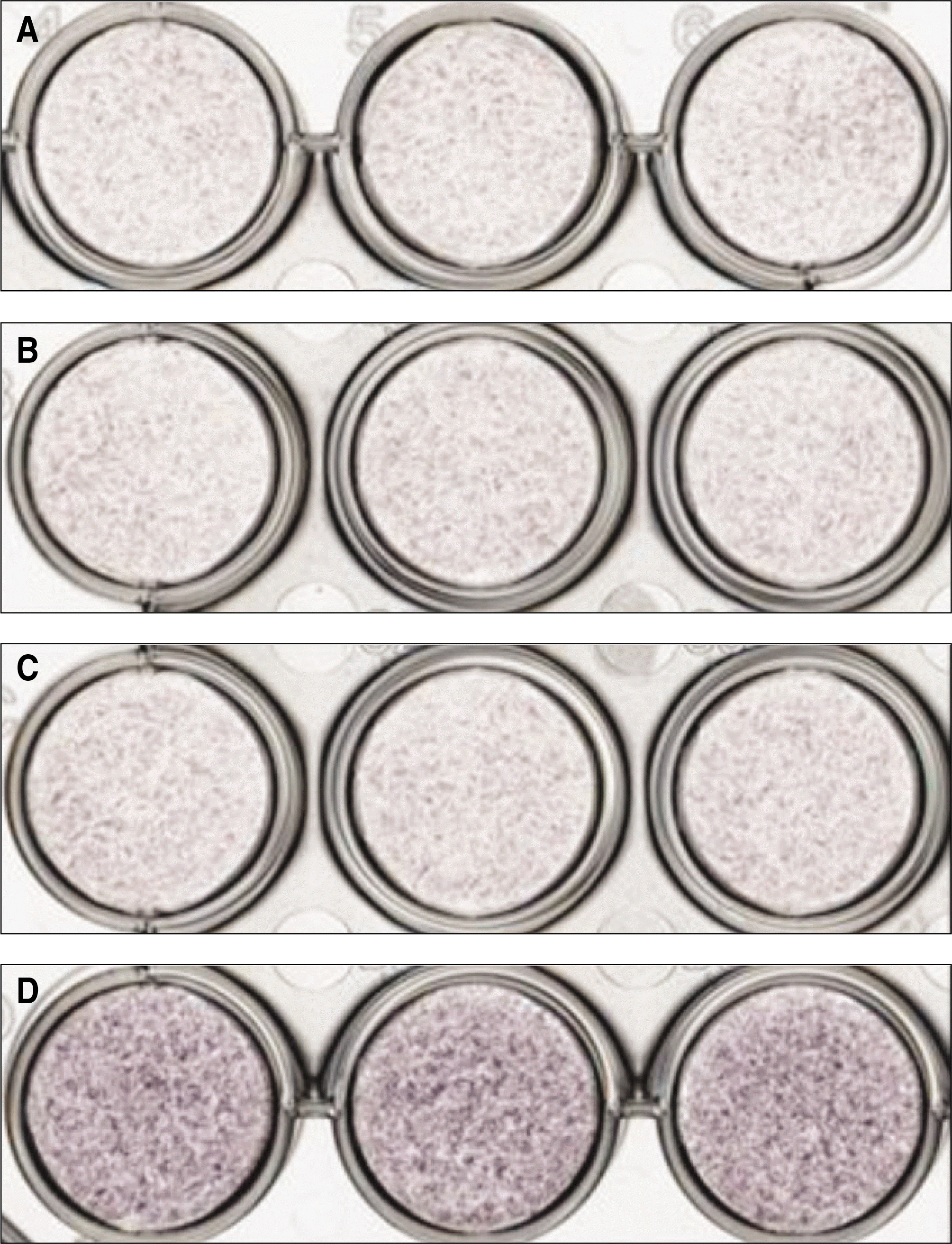
Fig. 2.
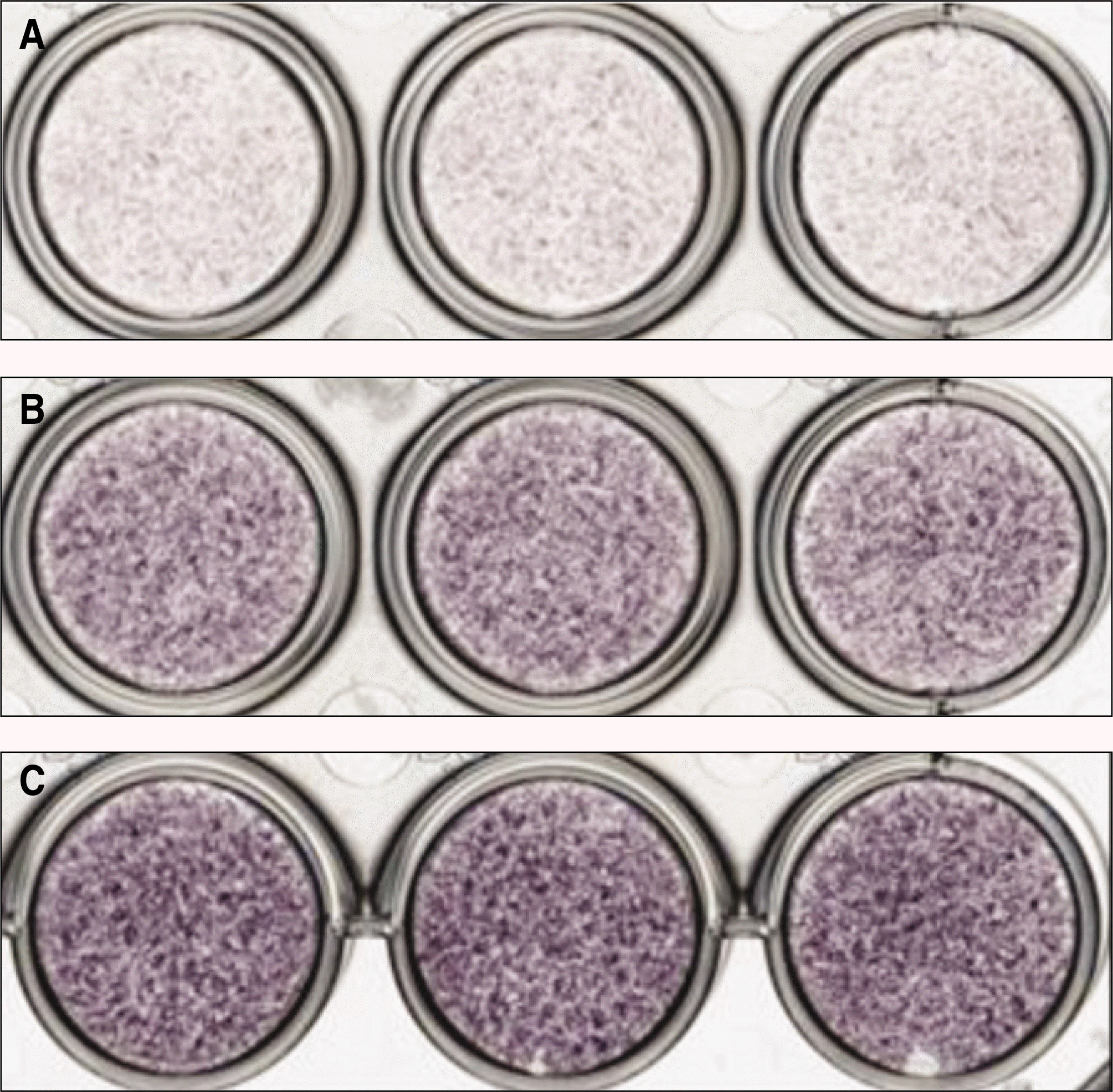
ALP expression in the periosteal-derived cells treated with IL-1βat 3 days of culture. A. Treatment of 0.01 ng/mL IL-1β. B. Treatment of 0.1 ng/mL IL-1β. C. Treatment of 1 ng/mL IL-1β. (ALP: alkaline phosphatase, IL: interleukin)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download