Abstract
Purpose
Impaired left ventricular (LV) global longitudinal strain (GLS) and the presence of microalbuminuria indicate early cardiac and renal dysfunction. We aimed to determine the relationships among 24-h ambulatory blood pressure (BP) variables, LV GLS, and urine albumin creatinine ratio (UACR) in hypertensive patients.
Materials and Methods
A total of 130 hypertensive patients (mean age 53 years; 59 men) underwent 24-h ambulatory BP monitoring, measurements of peripheral and central BPs, and transthoracic echocardiography. Patients with apparent LV systolic dysfunction (LV ejection fraction <50%) or chronic kidney disease were not included. LV GLS was calculated using two-dimensional speckle tracking, and UACR was analyzed from spot urine samples.
Results
In simple correlation analysis, LV GLS showed the most significant correlation with mean daytime diastolic BP (DBP) (r=0.427, p<0.001) among the various BP variables analyzed. UACR revealed a significant correlation only with night-time mean systolic BP (SBP) (r=0.253, p=0.019). In multiple regression analysis, daytime mean DBP and night-time mean SBP were independent determinants for LV GLS (β=0.35, p=0.028) and log UACR (β=0.49, p=0.007), respectively, after controlling for confounding factors. Daytime mean DBP showed better diagnostic performance for impaired LV GLS than did peripheral or central DBPs, which were not diagnostic. Night-time mean SBP showed satisfactory diagnostic performance for microalbuminuria.
The presence of target organ damage in hypertensive patients is a risk factor for major cardiovascular events.12 Early detection of subclinical target organ dysfunction allows clinicians to prevent progression to target organ damage. Previous studies have suggested that ambulatory blood pressures (BPs) predict future cardiovascular events better than office peripheral BPs.34 Although many studies have demonstrated that ambulatory BP variables are associated with subclinical structural or functional changes in the heart and kidneys in various hypertensive populations,567 the data regarding the association between daytime or night-time BPs and cardiac or renal damage are discrepant and inconclusive. Furthermore, cardiac and renal damage may not occur in parallel in each individual with mild hypertension.8
Most previous studies have used the presence of left ventricular hypertrophy (LVH) or increased left ventricular (LV) mass index as a surrogate indicator for target organ damage to the heart.9 Recently, LV global longitudinal strain (GLS) was shown to be a sensitive marker of subclinical LV dysfunction, and the independent prognostic implications of LV GLS have been verified in various cardiovascular diseases, including hypertension.101112 Microalbuminuria is known as an early marker of target organ damage in hypertension.13 Accordingly, in this study, we tested the hypotheses that 1) ambulatory BP variables would better correlate with LV GLS than would peripheral or central BPs and that the associations would be significant even in patients without LVH, that 2) ambulatory BP variables would better correlate with degrees of microalbuminuria than would peripheral or central BPs, and that 3) associations between ambulatory BP variables and early cardiac or renal dysfunction would differ in hypertensive patients without clinically apparent target organ damage.
This cross-sectional study included 130 patients with at least a 3-month history of uncomplicated arterial hypertension, according to current guidelines.14 Patients were enrolled from the outpatient clinic of a single tertiary center from March 2011 to December 2015. At initial enrollment, a complete clinical examination was performed to exclude secondary causes of hypertension. Past medical histories, including current hypertensive medications, were reviewed through electronic medical records. Exclusion criteria included diabetes mellitus, significant valvular disease (≥moderate severity), apparent LV systolic dysfunction (ejection fraction <50%), chronic kidney disease [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2], hypertrophic cardiomyopathy, previous history of stroke, previous history of myocardial infarction, unstable angina, atrial fibrillation, life threatening arrhythmia, secondary hypertensive disease, or neoplastic disease. Smokers were defined as current smokers.
Comprehensive cardiovascular assessment, including electrocardiogram, transthoracic echocardiography, 24-h ambulatory BP monitoring, measurements of peripheral and central BPs, and laboratory assessment, including fasting glucose, lipid profile, blood urea nitrogen, creatinine, and urine albumin creatinine ratio (UACR), was performed at initial enrollment. Written informed consent was obtained from all patients. The protocol was approved by our Institutional Review Board (IRB No. 2017-1681-001).
Peripheral systolic BP (SBP) and diastolic BP (DBP) measurements were performed automatically (Omron M4 Plus, Omron Healthcare Co. Ltd., Kyoto, Japan) at the brachial artery of the non-dominant arm in a relaxed seated position. Two BP measurements obtained at an interval of 5 minutes during the same visit were performed and averaged. Central SBP, DBP, and pulse pressure (PP) were assessed with pulse wave analysis of the radial artery using commercially available radial artery tonometry (SphygmoCor, AtCor Medical, Sydney, Australia).1516
Ambulatory BP monitoring was performed with an appropriately sized BP cuff on the non-dominant arm using a BP monitor (Model 90207; Spacelabs Healthcare; Snoqualmie, WA, USA) programmed to record the BP every 1 hour throughout a 24-h period. Patients were instructed to note the times of going to bed in the evening and waking up in the morning to calculate mean daytime and night-time BPs. Night-time BP was defined as the BPs from the time when the patient went to bed until the time he or she got out of bed, and daytime BP was defined as the BPs recorded during the rest of the day. Mean 24-h BP was calculated as the mean of all the readings throughout the 24-h period. The morning BP surge was calculated as follows: morning SBP–lowest night-time SBP.17 Nocturnal hypertension was defined as night-time mean SBP ≥120 mm Hg or DBP ≥70 mm Hg.
Each patient underwent a comprehensive transthoracic echocardiographic study using a Vivid 7 or Vivid 9 cardiovascular ultrasound system (GE Medical Systems, Horten, Norway) equipped with 2.5–3.5 MHz phased-array sector probes. Standard two-dimensional (2D) and Doppler measurements were performed per the recommendations of the American Society of Echocardiography guidelines.18 LVH was diagnosed according to the American Society of Echocardiography recommended formula for estimation of the LV mass index and indexed to body surface area (cutoff values for men LV mass index >115 g/m2 and for women >95 g/m2). From the apical window, mitral inflow velocities were traced, and the following variables were obtained: peak velocity of early diastolic mitral inflow (E), late diastolic mitral inflow (A), and deceleration time of the E velocity. Early diastolic mitral annular velocity (e′), late diastolic mitral annular velocity, and systolic mitral annular velocity (S′) were measured from the apical four-chamber view with a 2- to 5-mm sample volume placed at the septal corner of the mitral annulus.
Each patient underwent 2D-speckle echocardiography of the LV. Three consecutive cardiac cycles were recorded and averaged, and the frame rates were set to 60–80 frames/sec. LV GLS was measured by averaging all regional peak longitudinal strains from three apical views (apical 4-, 2-, and 3-chamber views). Standard deviations (SDs) of time to peak longitudinal strain (LS) from all segments were analyzed (SD of time to peak LS). LV global circumferential strain (GCS) was obtained from averaging all circumferential strains from short axis views of the mitral valve, papillary muscle, and LV apex level. The analysis was performed offline using customized software (EchoPAC PC, version 113; GE Medical Systems).
Early cardiac or renal dysfunction was defined by the presence of abnormal LV GLS or the presence of microalbuminuria, respectively. The presence of microalbuminuria was assessed by gender-specific UACR cut-off values (≥17 mg/g in men and ≥25 mg/g in women).19 Abnormal LV GLS was defined as a GLS absolute value less than 20% in accordance with current guidelines.20
Continuous variables are presented as means±SD, and categorical variables are presented as a percentage. Spearman's simple correlation analyses were performed to assess correlations with 24-h ambulatory BP variables with log UACR or LV GLS. Multiple linear regressions were performed to assess independent associations between LV GLS or log UACR with the 24-h ambulatory BP parameters, respectively. Covariates, including age, gender, and other pertinent covariates, were selected based on their association with each parameter (the threshold for inclusion in the multivariable models was set at a p value of <0.2). The performance of the predictive selected parameters was assessed using the area under the receiver operating characteristic (ROC) curve (AUC), and diagnostic performances were compared by means of Delong tests.21 A p-value <0.05 was regarded as statistically significant. Analyses were undertaken using SPSS 21.0 (IBM Corp., Armonk, NY, USA).
Interobserver and intraobserver variabilities were examined for LV GLS and GCS using intraclass and interclass correlation coefficients. Intra-observer variability for LV GLS and GCS parameters was analyzed for a group of twenty randomly selected subjects by one observed who repeated it twice. Inter-observed variability was assessed with the same data sets by two investigators who were unaware of each other's measurements. The intraobserver and interobserver interclass correlation coefficients were 0.97 [95% confidene interval (CI): 0.89–0.99] and 0.94 (95% CI: 0.29–0.98) for LV GLS and 0.94 (95% CI: 0.84–0.98) and 0.90 (95% CI: 0.75–0.96) for the LV GCS, respectively.
The mean age of the enrolled patients was 53±14 years and 59 (45%) were men. The incidence of comorbidities is described in Table 1; none of the patients were diagnosed with diabetes mellitus, atrial fibrillation, previous myocardial infarction, or stroke. Only 24 (18%) were diagnosed with dyslipidemia. Twenty-one patients (16%) had microalbuminuria and 33 (25%) showed LVH as target organ damage. Fifty-nine patients (45%) were drug naïve hypertensive patients. The most prevalent hypertensive medication was angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in 52 patients (40%).
Echocardiographic characteristics of the patients are described in Table 2. The mean LV mass index was 91.8±19.6 g/m2. The incidence of abnormal LV geometry was as follows: concentric LV remodeling in 11 (8.5%) patients, concentric LVH in 13 (10.0%) patients, and eccentric LVH in 20 (15.4%) patients. LV ejection fraction was within the normal range (68±6%); however, the mean value of LV GLS (−17.3±2.9 %) was not within the normal range.
Peripheral BP, central BP, and 24-h ambulatory BPs of patients revealed that the enrolled patients were mildly hypertensive as described in Table 3. The mean value of peripheral SBP was 146 mm Hg, while mean values of central SBP and 24-hr mean SBP were 134 mm Hg and 135 mm Hg, respectively. Thirty-nine (30%) patients revealed morning surges, and 86 patients (66%) met the criteria of nocturnal hypertension.
In a simple correlation analysis, LV GLS was significantly associated with peripheral SBP, DBP, central SBP, DBP, and all 24-h ambulatory BP parameters, while LV mass index did not show any significant association with any of the BP parameters (Fig. 1A and B, Table 3). Although the association of LV GLS with daytime mean DBP was significant in patients, regardless of the presence of LVH, the association was stronger in patients without LVH than in those with LVH (Fig. 1C and D). Log UACR showed a significant correlation only with night-time mean SBP (r=0.253, p=0.019) (Fig. 2, Table 3).
Multiple regression analysis revealed that LV GLS had an independent correlation with daytime mean DBP (β=0.35, p=0.028), even after adjustment for age, gender, usage of renin angiotensin aldosterone system (RAAS) blocker, LV mass index, LV ejection fraction, peripheral DBP, central DBP, and night-time mean DBP (Table 4). Log UACR showed an independent correlation with night-time mean SBP (β=0.49, p=0.007), even after adjustment for age, gender, usage of RAAS blocker, eGFR, peripheral SBP, central SBP, and daytime mean SBP (Table 4).
As shown in Fig. 3A, diagnostic performances of peripheral DBP, central DBP, daytime mean DBP, and night-time mean DBP for abnormal LV GLS were all satisfactory (peripheral DBP, AUC=0.706, p=0.006; central DBP, AUC=0.701, p=0.007; daytime mean DBP, AUC=0.803, p<0.001; night-time mean DBP, AUC=0.705, p=0.006). There was no significant difference among diagnostic performances of BP parameters for abnormal LV GLS, while daytime mean DBP showed the largest AUC (AUC: 0.803, 95% CI: 0.711–0.896, p<0.001) among four BP parameters (Fig. 3A). Evaluation of diagnostic performances for microalbuminuria indicated that only night-time mean SBP had a significant p value (AUC: 0.700, 95% CI: 0.580–0.824, p=0.004), while peripheral SBP, central SBP, and daytime mean SBP did not (Fig. 3B).
The principal findings in the present study are that 1) daytime mean DBP correlated better with LV GLS than did peripheral or central BPs, regardless of the presence of LVH, that 2) night-time mean SBP was the only parameter that showed a significant correlation with the degree of microalbuminuria, and that 3) associations between ambulatory BP variables and early cardiac or renal dysfunction differ in hypertensive patients without clinically apparent target organ damage. These data suggest that risk assessments using ambulatory BP variables will be useful for the prediction of early cardiac and renal dysfunction.
Current guidelines recommend assessing target organ damage in hypertensive patients, as they have independent prognostic significance and are associated with an increased cardiovascular mortality of >20% within 10 years.122 Previous studies showed that subclinical target organ damage, such as LVH as detected by echocardiography or microalbuminuria, are reliable cardiovascular risk predictors.232425 Subclinical LV dysfunction presenting with reduced LV GLS in hypertensive patients has been recently recognized.26272829 Saito, et al.26 reported that reduced LV GLS is independently associated with major adverse cardiovascular events. Therefore, patients with subclinical target organ damage are at high risk and should be treated more meticulously. A recent study reported that mechanical abnormalities of the LV in hypertensive patients with normal ejection fraction could be improved by targeted antihypertensive treatment.30 These findings suggest the importance of using adequate therapeutic monitoring parameters for subclinical target organ damage that may lead to improved clinical prognoses.
From the multivariate analysis in our study, daytime mean DBP was shown to be significantly associated with abnormal LV GLS, even after adjustment for age, gender, and peripheral and central BP parameters. From a simple correlation analysis, peripheral SBP and DBP showed significant correlations with LV GLS; however, daytime mean SBP and mean DBP showed stronger correlations. It is interesting that daytime mean DBP was independently associated with LV GLS, whereas daytime mean SBP showed less of an association. A previous study suggested a different clinical implication of ambulatory BP according to age, in which, in patients aged <60 years, ambulatory DBP provided significant incremental value for predicting a morbid cardiovascular event, whereas in patients aged >60 years, ambulatory PP was the best predictor of adverse events.31 In a Framingham study, DBP was the best predictor of coronary heart disease risk in men aged <45 years.32 Therefore, we believe the emphasis on daytime mean DBP over SBP should be interpreted with consideration of the mean age of our cohort. A progressive rise in both SBP and DBP in middle-aged populations correlates with the predominance of increased peripheral resistance, while increased PP due to a continued rise in SBP with a fall or plateau in DBP in older adults is associated with a predominance of arterial stiffness.33 Therefore, when monitoring early hypertensive patients at a relatively young age, DBP should be considered, as it reflects the main pathophysiology of hypertension in this age range. In addition, daytime mean DBP may better reflect peripheral resistance than mean SBP because it was steadier than SBP when the subjects engaged in daily activities.
A previous study by Sera, et al.34 reported stronger associations between impaired LV GLS and ambulatory BP parameters, compared to office BP parameters, in older adult patients. Our results also showed stronger correlation between abnormal LV GLS and ambulatory BP parameter than peripheral or central BP parameters. Also, our study population comprised younger hypertensive patients with a relatively low burden of comorbidity, compared to previous studies; therefore, confounding factors that could affect LV GLS were limited. Therefore, our results may strengthen previous studies by showing similar results in different populations and including central BP. Interestingly, the association with daytime BP and LV GLS was more significant in patients without LVH than in those with LVH (Fig. 1), suggesting that functional changes in the LV occur before structural changes of the LV and well reflects arterial loads. This finding demonstrates that LV GLS could be used for risk stratification of mild hypertensive patients without clinically significant target organ damage.
Night time mean SBP showed better diagnostic power for the presence of microalbuminuria, compared to the peripheral, central, and daytime BP parameters. Previous studies have addressed the predictive role of night-time BP in comparison with daytime BP in different populations of hypertensive patients;3536 however, one study presented contrary results.31 Our data supports a significant association of night-time mean SBP with subclinical renal damage in mild asymptomatic hypertensive patients. In this study, the superiority of night-time mean SBP for predicting UACRs over other BP parameters was demonstrated without using arbitrary dichotomous BP-related variables. The possible mechanism for the significant association of night-time BP and the presence of microalbuminuria could be explained by a blunted nocturnal BP fall from an imbalance of the autonomic nervous system favoring sympathetic over-activity, altered baroreceptor sensitivity, or nocturnal volume overload needing higher night-time BP to sustain natriuresis.3738
There are some limitations to this study. First, because of the cross-sectional nature of our study, we cannot distinguish cause from effect. Second, patients enrolled in this study were selected from a population with mild hypertension without comorbidities, including diabetes mellitus, atrial fibrillations, or coronary artery diseases. However, a lack of confounding comorbidities was beneficial when analyzing LV mechanics in this population with limited confounding factors. Third, we assessed soft endpoints for the presence of subclinical target organ damage; therefore, our results cannot be extrapolated for the incidence of hard cardiovascular endpoints. However, given that subclinical target organ damage is a significant risk factor for hard cardiovascular outcomes, treating asymptomatic patients with mild hypertension with risk stratifications based on associated subclinical target organ damage may be an effective strategy for reducing future cardiovascular events.
In conclusion, daytime mean DBP showed a significant association with LV GLS regardless of the presence of LVH. Night-time mean SBP better correlated with microalbuminuria than did peripheral or central BP in patients with mild hypertension. Ambulatory BP presented stronger associations with early cardiac and renal dysfunction than did peripheral or central BP, even in mild hypertensive patients without clinically apparent target organ damage. Therefore, assessment of ambulatory BP in mild hypertensive patients may help to guide BP-lowering treatment earlier and contribute to a reduction of future cardiovascular outcomes in the long term.
ACKNOWLEDGEMENTS
This study was supported by the grant “Research grant from Korean Society of Hypertension, 2014.”
References
1. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Blood Press. 2009; 18:308–347. PMID: 20001654.

2. Tocci G, Sciarretta S, Volpe M. Development of heart failure in recent hypertension trials. J Hypertens. 2008; 26:1477–1486. PMID: 18551026.

3. Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003; 348:2407–2415. PMID: 12802026.

4. Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005; 46:156–161. PMID: 15939805.
5. Wei FF, Li Y, Zhang L, Xu TY, Ding FH, Staessen JA, et al. Association of target organ damage with 24-hour systolic and diastolic blood pressure levels and hypertension subtypes in untreated Chinese. Hypertension. 2014; 63:222–228. PMID: 24246384.

6. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006; 19:243–250. PMID: 16500508.

7. Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000; 36:894–900. PMID: 11082163.

8. Palatini P, Graniero GR, Canali C, Santonastaso M, Mos L, Piccolo D, et al. Relationship between albumin excretion rate, ambulatory blood pressure and left ventricular hypertrophy in mild hypertension. J Hypertens. 1995; 13(12 Pt 2):1796–1800. PMID: 8903654.

9. Manyari DE. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990; 323:1706–1707. PMID: 2146505.

10. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014; 100:1673–1680. PMID: 24860005.

11. Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, et al. Paramount Investigators. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014; 63:447–456. PMID: 24184245.

12. Na HM, Cho GY, Lee JM, Cha MJ, Yoon YE, Lee SP, et al. Echocardiographic predictors for left ventricular remodeling after acute ST elevation myocardial infarction with low risk group: speckle tracking analysis. J Cardiovasc Ultrasound. 2016; 24:128–134. PMID: 27358705.

13. Pontremoli R, Nicolella C, Viazzi F, Ravera M, Sofia A, Berruti V, et al. Microalbuminuria is an early marker of target organ damage in essential hypertension. Am J Hypertens. 1998; 11(4 Pt 1):430–438. PMID: 9607381.

14. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, BÖhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013; 31:1281–1357. PMID: 23817082.
15. Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008; 21:1194–1202. PMID: 18787521.

16. Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004; 109:184–189. PMID: 14662706.

17. Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003; 107:1401–1406. PMID: 12642361.
18. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463. PMID: 16376782.

19. Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002; 13:1034–1039. PMID: 11912263.

20. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16:233–270. PMID: 25712077.

21. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.

22. Park S, Kario K, Park CG, Huang QF, Cheng HM, Hoshide S, et al. Characteristics On the ManagEment of Hypertension in Asia-Morning Hypertension Discussion Group (COME Asia MHDG). Target blood pressure in patients with diabetes: Asian perspective. Yonsei Med J. 2016; 57:1307–1311. PMID: 27593856.

23. Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging. 2012; 5:837–848. PMID: 22897998.

24. Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, et al. Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT) Investigators. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004; 110:2809–2816. PMID: 15492322.

25. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004; 110:921–927. PMID: 15302780.

26. Saito M, Khan F, Stoklosa T, Iannaccone A, Negishi K, Marwick TH. Prognostic implications of LV strain risk score in asymptomatic patients with hypertensive heart disease. JACC Cardiovasc Imaging. 2016; 9:911–921. PMID: 27344417.
27. Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009; 2:382–390. PMID: 19808626.
28. Imbalzano E, Zito C, Carerj S, Oreto G, Mandraffino G, Cusmà-Piccione M, et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography. 2011; 28:649–657. PMID: 21676016.

29. Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. Concentric left ventricular hypertrophy brings deterioration of systolic longitudinal, circumferential, and radial myocardial deformation in hypertensive patients with preserved left ventricular pump function. J Cardiol. 2010; 55:23–33. PMID: 20122545.

30. Cheng S, Shah AM, Albisu JP, Desai AS, Hilkert RJ, Izzo J, et al. Reversibility of left ventricular mechanical dysfunction in patients with hypertensive heart disease. J Hypertens. 2014; 32:2479–2486. PMID: 25232755.

31. Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation. 2001; 104:783–789. PMID: 11502703.

32. Kannel WB, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol. 1971; 27:335–346. PMID: 5572576.
33. Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997; 96:308–315. PMID: 9236450.
34. Sera F, Jin Z, Russo C, Lee ES, Schwartz JE, Rundek T, et al. Relationship of office and ambulatory blood pressure with left ventricular global longitudinal strain. Am J Hypertens. 2016; 29:1261–1267.

35. Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008; 21:443–450. PMID: 18292756.

36. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011; 57:3–10. PMID: 21079049.

37. Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Bombelli M, Cuspidi C, et al. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension. 2008; 52:925–931. PMID: 18779438.

38. Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006; 48:527–533. PMID: 16940213.

Fig. 1
Associations between daytime mean DBP and subclinical LV dysfunction. (A) Association between daytime mean DBP and LV mass index in overall patients. (B) Association between daytime mean DBP and LV GLS in overall patients. (C) Association between daytime mean DBP and LV GLS in patients without LVH. (D) Association between daytime mean DBP and LV GLS in patients with LVH. DBP, diastolic blood pressure; LV, left ventricle; GLS, global longitudinal strain; LVH, left ventricular hypertrophy.
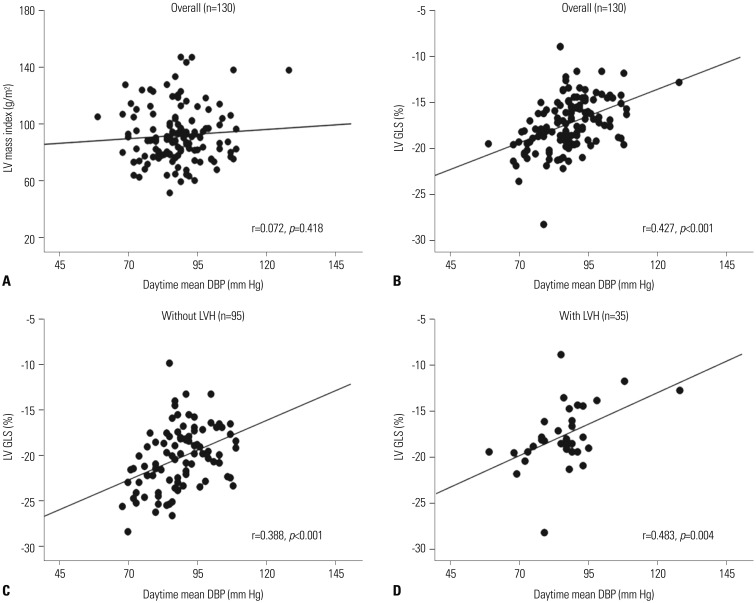
Fig. 2
Associations between night-time mean SBP and log UACR. SBP, systolic blood pressure; UACR, urine albumin creatinine ratio.
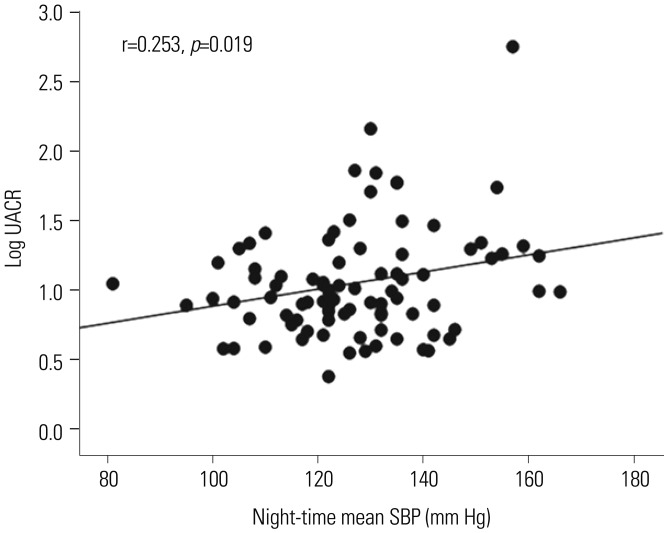
Fig. 3
Diagnostic performances for subclinical LV dysfunction (A) and presence of microalbuminuria (B). LV, left ventricle; GLS, global longitudinal strain; DBP, diastolic blood pressure; SBP, systolic blood pressure; AUC, area under the receiver operating characteristic curve.
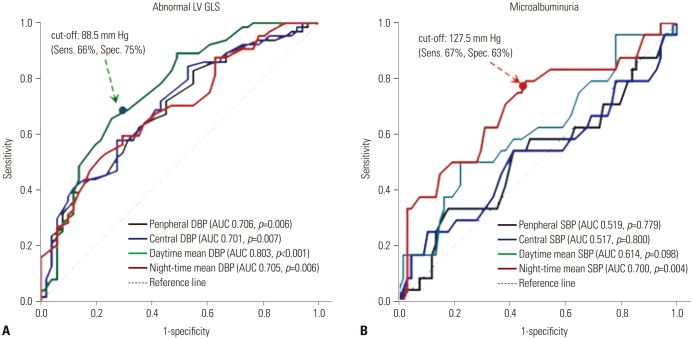
Table 1
Baseline Characteristics of the Study Population
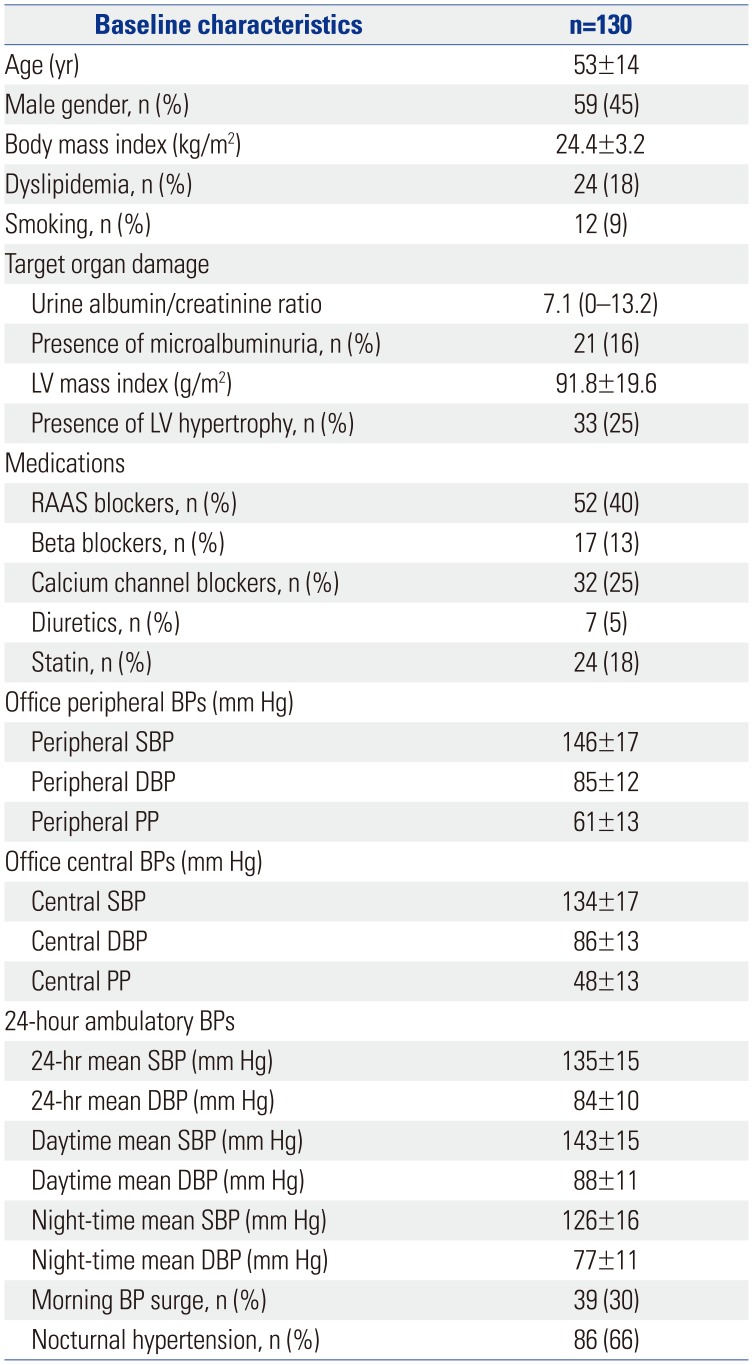
Table 2
2D, Doppler, and Speckle Tracking Echocardiography
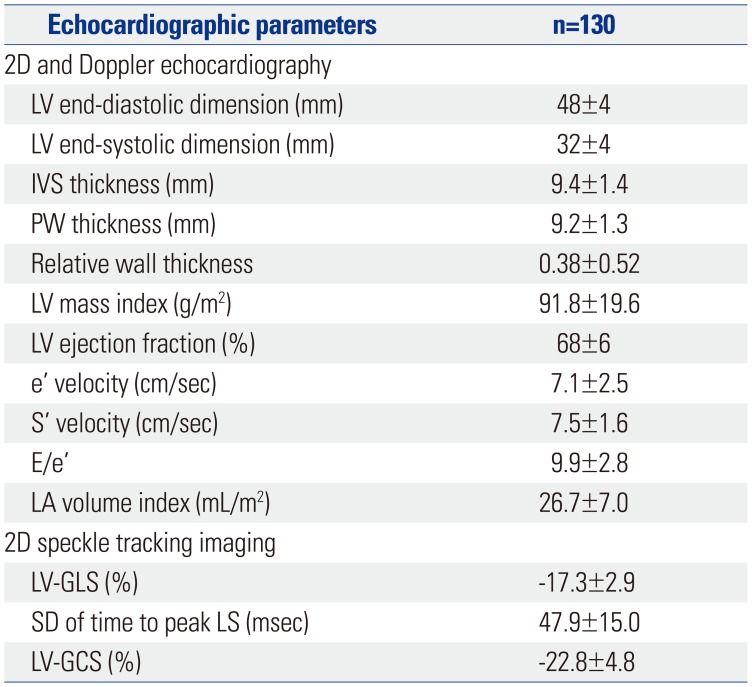
Table 3
Simple Correlations between Blood Pressures and Target Organ Dysfunction
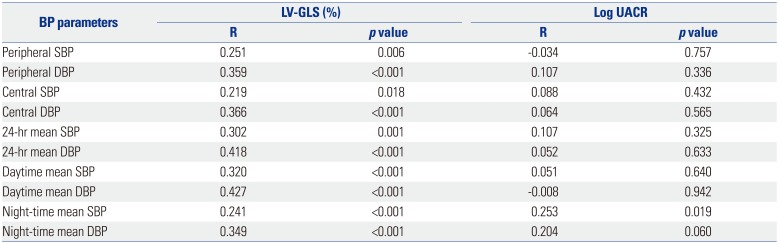
Table 4
Determinants for Early Cardiac and Renal Dysfunction




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download