Abstract
Purpose
The fourth state of matter, plasma is known as an ionized gas with electrons, radicals and ions. The use of non-thermal plasma (NTP) in cancer research became possible because of the progresses in plasma medicine. Previous studies on the potential NTP-mediated cancer therapy have mainly concentrated on cancer cell apoptosis. In the present study, we compared the inhibitory effect of NTP on cell migration and invasion in the oral squamous cancer cell lines.
Materials and Methods
We used oral squamous cancer cell lines (SCC1483, MSKQLL1) and different gases (N2, He, and Ar). To investigate the mechanism of plasma treatment, using different gases (N2, He, and Ar) which induces anti-migration and anti-invasion properties, we performed wound healing assay, invasion assay and gelatin zymography.
Oral squamous cell carcinoma (OSCC) accounts for ~3% of all cancer diagnoses, and is one of the most common head-and-neck cancer (HNC).1 There has been a lot of progress in OSCC treatment protocols including surgery, radiotherapy and chemotherapy, nevertheless, the long-term survival rate of patients with OSCC unchanged over the past decade.12 Therefore, many studies have been conducteded for OSCC treatment. In these studies, we found that a combination of inter-reliant processes, including cell migration, invasion, surface adhesion, and extracellular matrix (ECM) degradation by matrix metalloproteinases (MMPs), resulted in an impact on the invasive and metastatic mechanism by complicated mechanisms.34 Tumor metastasis is one of major cause of mortality in cancer patients.5 Thus, preventing cancer cell migration and invasion, which induce tumor metastasis, is crucial for cancer treatment.
Non-thermal plasma (NTP) is an ionized gas consisting of electrons, ions, neutral atoms, radicals, and UV photons; also called the fourth state of matter after solid, liquids and gases.6 NTP is known as a novel treatment method for various cancers including lung cancer, melanoma and colorectal cancer,78910 and we previously reported that NTP induced apoptosis in HNC via the ATM/p53 signaling pathway,11 and caused cell death through mitochondrial reactive oxygen species by mitogen-activated protein kinase.12 It has also been reported that NTP suppresses the invasion of thyroid papillary cancer cell through cytoskeletal modulation.4 In addition, we reported that NTP induced delayed tumor invasion and growth arrest in colorectal and thyroid cancer cells.412 The anti-cancer effect of NTP in these instances was explained mainly by apoptosis due to the increase in reactive oxygen species.13
Many studies showed that biological effects of NTP vary by their gas types, their combinations, treatment conditions, and treated cell types.1314151617 Different plasma gas sources were used depending on various research groups and indications including anti-infection,1819 wound healing,20 and anticancer treatment.312 However, the inhibitory effects of NTP composed of different gases on cancer migration and invasion have not been directly compared until now. The purpose of this study is to compare the emission spectra between N2, He, and Ar, and the NTP suppressing effects on cell migration, cell invasion and apoptotic cell death in OSCC according to the type of gas. As far as we are aware of this is the first study comparing the anti-cancer effects on cell migration and invasion according to NTP gas type.
Two HNC cell lines originating from human oral cancer (MSKQLL1,SCC1483) were provided by Prof. Se-Heon Kim (Yonsei University, Korea). MSKQLL1 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEN): Nutrient Mixture F-12 (DMEM/F12; GIBCO, Carlsbad, CA, USA). whereas SCC1483 cells were cultured in Minimum Essential Medium (MEM; GIBCO, Carlsbad, CA, USA). The cells were grown in MEM with 10% fetal bovine serum and 1% penicillin-streptomycin, respectively. The cultured cells were maintained at 37℃ in a humidified atmosphere with 5% CO2/95% air.
We used a micro-nozzle plasma jet system. The microplasma jet nozzle is made up of a plasma generation module and its cases. The schematic view of its nozzle is shown on Supplementary Fig. 1A (only online). The main components of plasma generation module are a Ni-Co alloy electrode, a glass insulator, and an electrode ring. The Ni-Co alloy electrode and glass insulator were fabricated simultaneously by micromachining technology. Supplementary Fig. 1B (only online) shows the fabrication process of Ni-Co alloy electrode. A Cr/Au film was deposited on a glass wafer as a seed layer for electroplating. The 105 holes were arrayed along concentric circles on the Ni-Co alloy electrode and glass insulator, and the diameter and the depth of holes were 300 µm and 100 µm, respectively. Cells were grown up to 90%– confluence and exposed to NTP (He, Ar, and N2) 3 cm away from the nozzle for 1, 3, and 5 min. The gas flow rate was maintained at 8 L/min, and voltages were 15 KV and 20 kHz, respectively. We developed and produced NTP with a designed dielectric barrier discharge type to generate a homogenous NTP jet for biomedical research applications, as described previously.2122
Quantitative apoptotic cell death by plasma was detected using the Annexin V-propidium iodide (PI) apoptosis detection kit I according to the manufacturer's protocol (BD Biosciences, Bedford, MA, USA), as described previously.12 The cells were treated with various gas device (Ar, N2, and He) NTP for 1, 3, and 5 min and then incubated further for 24 h. The cells were harvested, washed with cold phosphate-buffered saline (PBS), and stained with Annexin V-fluorescein isothiocyanate and PI at room temperature for 15 mins in the dark. The early and late apoptosis were quantified according to the manufacturer's instructions. Apoptosis was detected using a FACS Aria system (BD Biosciences), with the excitation and emission settings of 488 and 530 nm, respectively.
For wound healing assasy, inducible MSKQLL1 and SCC1483 cells were plated on 12-well plates and grown to confluency (>90%), followed by serum starvation for 24 h. Wounds were generated by using a sterile 200-mL pipette tip and washing with PBS. The cells were then exposed to various gases (N2, Ar, and He) of NTP for 3 min. Each experiment was performed in triplicate. The wound on the captured image was automatically recognized and measured by Metamorph® NX image software (Molecular Devices, Sunnyvale, CA, USA) and eluate of crystal violet staining was measured under a light microscopy.
The invasion ability of each cancer cell line was evaluated using Transwell (24-well) chambers (pore size 8 µm, Costar, Cambridge, MA, USA), as described previously.7 Initially, type I collagen (8 µg/filter) was dissolved in 100 µL of medium and poured into the upper part of the polyethylene filter (pore size, 8 µm). The wells were coated overnight in a laminar flow hood. Then, 1×104 cells (in 100 µL of culture medium) were added to the top of the filter in the upper transwell chamber. The chamber was incubated for 24 h in 5% CO2 at 37℃. Finally, attached cells in the lower section (invading cells) were stained with hematoxylin and eosin (H&E) and counted in four representative fields by light microscopy (×200 magnification).
MMP-2/9 activity was examined using gelatin zymography as described previously.17 The cells were treated with He, Ar, or N2 plasma for 5 min and incubated for an additional 24 h. The supernatant (100 µL) from each sample was mixed with 1 µL of 100 mM APMA (4-aminophenylmercuric acetate, Sigma-Aldrich, St. Louis, MO, USA) and the samples were activated for 1 h at 37℃ incubator. Next, each sample was placed in sample buffer (without mercaptoethanol) for 10 min and electrophoresed in sodium dodecyl sulfate (SDS) page gelatin gels at 125 V for 120 min at room temperature using a Novex Xcell II system (Thermo Scientific, Waltham, MA, USA). The gels were incubated in renaturation buffer for 60 min at room temperature, followed by incubation for 18 h in 100 mL of Novex zymogram developing buffer (Thermo Scientific) at 37℃ with orbital shaking. The gels were then stained for 3 h with Coomassie brilliant blue. After decolorization in 400 mL of methanol, 100 mL of acetic acid, and 500 mL of distilled water, images were taken using an image analyzer.
For Western blot, reduced protein samples were lysed by RIPA buffer containing 150 mM NaCl, 1.0% nonidet-P 40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0, and protease inhibitor cocktail (Roche Applied Science, Vienna, Austria; pH 7.4), as described previously.12 The following antibodies were used for Western blot analysis: anti-focal adhesion kinase (FAK), -integrin, -Paxillin, and -α-tubulin (1:1000; Cell Signaling Technology, Danvers, MA, USA).
After culture on a microscope cover glass (Thermo Scientific), the cells were treated with He, Ar, and N2 of NTP, and incubated for an additional 24 h. The cells were fixed with 4% formaldehyde and blocked in bovine serum albumin in 5% PBS for 45 min. Slides were then incubated with a polyclonal rabbit anti-p-FAK antibody (1:50; Cell Signaling Technology) for 2 h, washed with PBS and incubated with an Alexa 546-labeled goat anti-rabbit antibody (1:250; Molecular Probe, Eugene, Oregon, CA, USA) for 45 min. After rinsing in PBS, Hoechst 33258 (Molecular Probe) was added to slides for 15 min to counterstain nuclei. Slides were washed with PBS, mounted with Vectashield (Vector laboratories, Inc., Burlingame, CA, USA), and then visualized using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
All data from three independent experiments are expressed as mean±SD. A p<0.05 was considered statistically significant (*p<0.05; **p<0.01; ***p<0.001).
The means for the different groups were compared using One-way analysis of variance, followed by post hoc Tukey's test, performed using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA).
To estimate various species produced from the plasma jet, we performed optical emission spectrum analyses. Optical emission spectroscopy was conducted from 280 nm to 920 nm wavelengths by optical emission spectroscope (SV 2100, K-MAC, Daejeon, Korea) (Fig. 1), and the optical emissions of three gases were compared: N2, He, and Ar from a NTP jet at 5 L/min. The N2 emission spectrum (Fig. 1A) was settled mostly by the presence of nitrogen (N) species, including N2 second (290–410 nm), first positive systems (600–700 nm) and N2+ first negative system (410–600 nm). Moreover, considerably reactive radicals were tied to oxygen, such as oxygen ions (O2+) at 500–600 nm and weak atomic N at 747, 822, and 868 nm, were observed. The Ar and He NTP emission spectra (Fig. 1B and C) showed that the respective plasma species tied to argon and helium were superior. From Ar and He NTP in the emission spectra, we detected hydroxyl radicals and excited oxygen atoms. However, the intensities of oxygen atoms in Ar and He NTP were weaker than in N2 NTP. These findings imply that N2 NTP would be more effective for biomedical applications than He or Ar NTP.
It has been previously demonstrated that NTP induce apoptotic cancer cell death.12 Therefore, to estimate the effects of NTP on apoptotic cell death by different gas types, we measured annexin V-PI staining according to the processing time of each gas (Fig. 2). No significant SCC1483 and MSKQLL1 cell death was induced by 1 and 3 min NTP treatment with various gas sources (N2, Ar, and He). However, significant cell death in both SCC1483 (Fig. 2A) and MSKQLL1 (Fig. 2B) cell lines was noted in all three gas plasmas after 5 min treatments.
Our previous studies showed that NTP treatement with the mixture of O2 and He induces cellular morphological change and reduces tumor cell migration.3 To estimate the effect of NTP on cell migration in OSCC by gas type, we performed a wound-healing assay (Fig. 3): NTP treatment for 3 min was used, because NTP treatment for 5 min induced significant cancer cell death (Fig. 2). Thus, cells were exposed to N2, He, and Ar NTP for 3 min and incubated for 24 h. Results showed that that NTP with all three gases significantly suppressed the migration of SCC1483 (Fig. 3A) and MSKQLL1 (Fig. 3B) cells across the denuded zone compared with control group. Furthermore, N2 NTP significantly suppressed the migration of both cell lines compared with He and Ar NTP.
To elucidate the suppressive effect of NTP induced by each gas on cellular invasion, we performed an invasion assay (Fig. 4). Twenty-four hours after incubation, H&E-stained OSCC cells were placed on the undersurface of the membrane. NTP treatment reduced the number of invading cells compared with gasonly treatment. It should be noted that N2 NTP among N2, He, and Ar NTP showed the most deterrent effect on cell invasion (Fig. 4A and B). In order to understand the mechanism of suppression effect of NTP on tumor invasion in vitro, we performed the gelatin zymography. Fig. 4C shows visible changes in MMP-2/9 activity in NTP-treated SCC1483 and MSKQLL1 cells (Fig. 4C), with the most significant reduction by N2 NTP among the gases.
FAK, integrin, and paxillin are well known proteins involved in cell migration and invasion.23 Thus, to elucidate the suppressive effect of NTP on migration, the expressions of T-FAK, p-FAK, integrin β3, t-paxillin, and p-paxillin were examined by western blot anlysis. As shown in Fig. 5A, the levels of p-FAK and integrin β3 in N2 NTP treated cells were significantly lower than those in He and Ar NTP in both cell lines. Moreover, immunocytochemistry confirmed that intracellular distribution of p-FAK underwent the most significant alteration in N2 NTP-treated SCC1483 and MSKQLL1 cells (Fig. 5B and C), respectively.
Recently, many research groups are actively involved in plasma medicine, and NTP has already been used widely in industrial and medical applications. According to previous studies, NTP is a novel therapeutic method which can induce cell death and inhibit cell migration and invasion in various cancers including HNC,21112 and many studies showed the anticancer effect of NTP via various mechanisms including cell cycle arrest and apoptosis induction.7810 We previously, reported that NTP exert a tumor suppression by inhibiting migration and invasion of cancer cell.4 However, the results were not reproducible due to different experimental system conditions.111224 In this study, we compared the NTP effects on cancer cell survival, migration, and invasion with different gases (N2, He, and Ar). To our best knowledge, it is the first time to explore optimized NTP condition which induces the best anticancer effect in OSCC cells.
To optimize the time of NTP treatment, we performed annexin V-PI staining after 1, 3, and 5 min of NTP treatment using N2, Ar, and He as gas sources. Fine min NTP treatment by all the three gases induced significant apoptotic cell death in both SCC1483 and MSKQLL1 cell lines. Therefore, to clearly evaluate the effect on cancer cell migaration and invasion, we selected 3 min NTP treatment condition.
MMPs are main members of proteases to degrade the ECM components. MMP activity is associated with tumor growth and metastasis. MMP-2/9 are highly expressed in colon, lung, prostate and breast cancer, and increased MMP-2/9 activity is due to the activation of FAK/ERK signaling.252627 Control of MMP is closely related to reducing the cancer cell invasion. In the present study, to compare the NTP suppression effect on tumor invasion by gas type in vitro,23 gelatin zymography was performed to confirm the MMP-2/9 activity, and the results of the zymography showed that NTP treatment by all the gases reduced MMP-2/9 expressions. In particular, N2 NTP induced the decrease more than other gases.
We also observed the inhibitory effect of NTP by various gases on OSCC cell invasion via the FAK and MMP system. FAK is a non-receptor tyrosine kinase whose expression increased in various invasive and metastatic cancer including OSCC.28 FAK signalings are also correlated to tumor invasion during cancer metastasis by ECM degradation.29 The connection of FAK and MMP-2/9 pathway has been shown previously;30 FAK promotes cell migration via the process of combination with Src and subsequent paxillin phosphorylation.31 In cancer cells, the inhibition of FAK decreases MMP-9 secretion.32 Our present results are in agreement with earlier studies, since the cell migration was inhibited by suppression of FAK and paxillin signaling after NTP treatment with various gases, even though the most prominent results were induced by N2 NTP.
In summary, N2 NTP suppressed OSCC cell migration and invasion more strongly than the other two gases, and most potently reduced FAK, integrin β3, and paxillin expression, which play key roles in tumor migration. N2 NTP also significantly attenuated the activity of MMP-2/9, suggesting that N2 might be more useful gas than Ar or He NTP for cancer treatment by NTP.
Figures and Tables
Fig. 1
Optical emission spectrum (OES) of plasma jet according to gases (A) OES of NTP induced by N2 gas, (B) He gas, and (C) Ar gas. NTP, non-thermal plasma.

Fig. 2
Cell death after 1, 3, and 5 min NTP treatment according to gases (N2, He, or Ar). (A) Annexin V-PI assay and quantification of SCC1483. Bar graph: mean±SD of 3 independent experiments. *p<0.05, ***p<0.001. NTP, non-thermal plasma; PI, propidium iodide.
*p<0.05. NTP, non-thermal plasma; PI, propidium iodide.

Fig. 3
Wound healing assay of 3 min NTP treatment according to gases (N2, He, or Ar) on (A) SCC1483 and (B) MSKQLL1. Data represents mean±SD of three independent experiments. *p<0.05, ***p<0.001. NTP, non-thermal plasma.
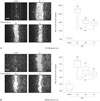
Fig. 4
Invasion assay of 3 min NTP treatment according to gases (N2, He, or Ar) on SCC1483 (A) and MSKQLL1 (B). Data represent mean±SD of three independent experiments. **p<0.01, ***p<0.001. NTP, non-thermal plasma.
(C) Gelatin zymography of 3 min NTP treatment according to gases (N2, He, or Ar) for MMP-2/9 on SCC1483 and MSKQLL1. *p<0.05, ***p<0.001. NTP, non-thermal plasma; MMPs, matrix metalloproteinases.
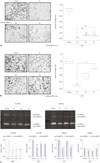
Fig. 5
(A) Western blots of p-focal adhesion kinase (FAK), FAK, Integrin (b3), p-paxillin, paxillin, and uncleaved caspase-3 after 3 min NTP treatment according to gases (N2, He, or Ar). (B) Immunocytochemical assay for p-FAK after 3 min NTP treatment according to gases (N2, He, or Ar) in SCC1483 and (C) MSKQLL1. Scale bar=10 µm. Each figure is representative of three experiments with triplicates. NTP, non-thermal plasma.
ACKNOWLEDGEMENTS
This research was supported by the Bio & Medical Technology Development Program (2012M3A9B2052870) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2015R1A2A1A01002968).
References
2. Chang JW, Kang SU, Shin YS, Seo SJ, Kim YS, Yang SS, et al. Combination of NTP with cetuximab inhibited invasion/migration of cetuximab-resistant OSCC cells: Involvement of NF-κB signaling. Sci Rep. 2015; 5:18208.

3. Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009; 185:357–370.

4. Chang JW, Kang SU, Shin YS, Kim KI, Seo SJ, Yang SS, et al. Non-thermal atmospheric pressure plasma inhibits thyroid papillary cancer cell invasion via cytoskeletal modulation, altered MMP-2/-9/uPA activity. PLoS One. 2014; 9:e92198.

5. Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, et al. EMT and tumor metastasis. Clin Transl Med. 2015; 4:6.

6. Moreau M, Orange N, Feuilloley MG. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008; 26:610–617.

7. Kim CH, Kwon S, Bahn JH, Lee K, Jun SI, Rack PD, et al. Effects of atmospheric nonthermal plasma on invasion of colorectal cancer cells. Appl Phys Lett. 2010; 96:243701.

8. Kim CH, Bahn JH, Lee SH, Kim GY, Jun SI, Lee K, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010; 150:530–538.

9. Panngom K, Baik KY, Nam MK, Han JH, Rhim H, Choi EH. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013; 4:e642.

10. Sensenig R, Kalghatgi S, Cerchar E, Fridman G, Shereshevsky A, Torabi B, et al. Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann Biomed Eng. 2011; 39:674–687.

11. Chang JW, Kang SU, Shin YS, Kim KI, Seo SJ, Yang SS, et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch Biochem Biophys. 2014; 545:133–140.

12. Kang SU, Cho JH, Chang JW, Shin YS, Kim KI, Park JK, et al. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014; 5:e1056.

13. Wang M, Holmes B, Cheng X, Zhu W, Keidar M, Zhang LG. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS One. 2013; 8:e73741.

14. Volotskova O, Shashurin A, Stepp MA, Pal-Ghosh S, Keidar M. Plasma-controlled cell migration: localization of cold plasma-cell interaction region. Plasma Med. 2011; 1:85–92.

15. Seo YS, Mohamed A-AH, Woo KC, Lee HW, Lee JK, Kim KT. Comparative studies of atmospheric pressure plasma characteristics between He and Ar working gases for sterilization. IEEE Trans Plasma Sci. 2010; 38:2954–2962.

16. Sagawa T, Takayama T, Oku T, Hayashi T, Ota H, Okamoto T, et al. Argon plasma coagulation for successful treatment of early gastric cancer with intramucosal invasion. Gut. 2003; 52:334–339.

17. Esposito AR, Kamikawa CM, Lucchesi C, Ferreira BMP, Duek EAdR. Benefits of oxygen and nitrogen plasma treatment in Vero cell affinity to poly(lactide-co-glycolide acid). Mater Res. 2013; 16:695–702.

18. Watts AE, Fubini SL, Vernier-Singer M, Golkowski C, Shin S, Todhunter RJ. In vitro analysis of nonthermal plasma as a disinfecting agent. Am J Vet Res. 2006; 67:2030–2035.

19. Ermolaeva SA, Sysolyatina EV, Kolkova NI, Bortsov P, Tuhvatulin AI, Vasiliev MM, et al. Non-thermal argon plasma is bactericidal for the intracellular bacterial pathogen Chlamydia trachomatis. J Med Microbiol. 2012; 61(Pt 6):793–799.

20. Kramer A, Lindequist U, Weltmann KD, Wilke C, von Woedtke T. Plasma Medicine - its perspective for wound therapy. GMS Krankenhhyg Interdiszip. 2008; 3:Doc16.
21. Ahn HJ, Kim KI, Hoan NN, Kim CH, Moon E, Choi KS, et al. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS One. 2014; 9:e86173.

22. Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011; 6:e28154.

23. Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Das Gupta TK. Molecular prognostic markers in intermediate-thickness cutaneous malignant melanoma. Cancer. 1999; 85:375–382.

24. Tuhvatulin AI, Sysolyatina EV, Scheblyakov DV, Logunov DY, Vasiliev MM, Yurova MA, et al. Non-thermal plasma causes p53-dependent apoptosis in human colon carcinoma cells. Acta Naturae. 2012; 4:82–87.

25. Kohn EC, Liotta LA. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995; 55:1856–1862.
26. Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001; 84:1488–1496.

27. Yang YN, Wang F, Zhou W, Wu ZQ, Xing YQ. TNF-α stimulates MMP-2 and MMP-9 activities in human corneal epithelial cells via the activation of FAK/ERK signaling. Ophthalmic Res. 2012; 48:165–170.

28. Rosado P, Lequerica-Fernández P, Peña I, Alonso-Durán L, de Vicente JC. In oral squamous cell carcinoma, high FAK expression is correlated with low P53 expression. Virchows Arch. 2012; 461:163–168.

29. Lu Z, Lu N, Li C, Li F, Zhao K, Lin B, et al. Oroxylin A inhibits matrix metalloproteinase-2/9 expression and activation by up-regulating tissue inhibitor of metalloproteinase-2 and suppressing the ERK1/2 signaling pathway. Toxicol Lett. 2012; 209:211–220.

30. Kwiatkowska A, Kijewska M, Lipko M, Hibner U, Kaminska B. Downregulation of Akt and FAK phosphorylation reduces invasion of glioblastoma cells by impairment of MT1-MMP shuttling to lamellipodia and downregulates MMPs expression. Biochim Biophys Acta. 2011; 1813:655–667.

31. Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004; 6:154–161.

32. Shibata K, Kikkawa F, Nawa A, Thant AA, Naruse K, Mizutani S, et al. Both focal adhesion kinase and c-Ras are required for the enhanced matrix metalloproteinase 9 secretion by fibronectin in ovarian cancer cells. Cancer Res. 1998; 58:900–903.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download