INTRODUCTION
Osteoarthritis (OA) is a major public health issue that is responsible for chronic disability worldwide. Its incidence is expected to increase as the populations of many countries age.12 Although a broad range of mechanical and biochemical factors contribute to the development of OA, the primary cause is still unknown and optimal treatment remains elusive. Recent studies have demonstrated the importance of hypoxia-inducible factor-2 (HIF-2α) and vascular endothelial growth factor (VEGF) in the pathogenesis of OA.34 HIF-2α and VEGF are expressed by chondrocytes and are involved in the pathogenesis of OA,56 however, the relationship between these two factors and with OA is unclear.
In this study, we explored whether the expression of HIF-α and VEGF was related to the radiological findings in knee OA. Thus, we examined the expression of HIF-2α and VEGF in cartilage samples from the knee joints of patients with OA and analyzed the relationship between these two factors with respect to radiographic disease severity.
MATERIALS AND METHODS
Patients
From January 2012 to September 2014, 45 patients evaluated at the Department of Orthopedics and diagnosed with knee OA according to the American College of Rheumatology classification criteria were enrolled in the study. All of the patients had painful knees with joint effusion, as determined on physical examination. This study was approved by the Ethics Committee of Renmin Hospital, Wuhan University, China, and all patients provided written informed consent prior to their participation in the study.
Radiographic assessment
Each participant underwent radiography of the diseased knee. Imaging was performed with the patient standing on both legs with the knees fully extended. The X-ray beam was centered at the level of the joint. Disease severity was assessed using the Kellgren and Lawrence (KL) grading system.7 Scoring was based on the changes observed on conventional weight-bearing anterior-posterior radiographs of the affected knee in extension: grade 0 (normal findings), no X-ray changes; grade 1 (questionable), doubtful narrowing of the joint space and possible osteophytic lipping; grade 2 (mild), definite osteophytes and possible joint-space narrowing; grade 3 (moderate), multiple moderate osteophytes, definite joint-space narrowing, bone sclerosis, and possible deformity of the bone contour; and grade 4 (severe), large osteophytes, marked joint-space narrowing, severe sclerosis, and deformity of the bone contour. Controls were defined as having neither hip OA nor knee OA, as determined radiographically and indicated by KL grades of 0 for both hips and both knees. In patients with OA of both knees, the grade of the worst affected knee of each patient was used in the data analysis.
Hematoxylin and eosin and safranin-O/fast-green staining
Cartilage specimens were collected from 29 patients (31 knees) who underwent total knee replacement due to severe medial OA of the knee, 16 patients who underwent knee arthroscopy, and 5 patients with traumatic joints. The damaged cartilage was evaluated by two observers. The patients did not significantly differ in age, body mass index, or disease duration. Qualitative macroscopic evaluation of the injured cartilage was performed by one investigator. Cartilage damage was reported according to a semi-quantitative grading table modified from the human data reported in the literature and based on a scale of 0–4: 0=normal; 1=surface striations or tears; 2=bucket handle or transverse tears; 3=fibrillation of the entire cartilage, protrusion; 4=either loss of cartilage or ulcer.
The specimens were dissected and fixed for 48 h in neutral buffered 4% formaldehyde in 0.1 M sodium phosphate buffer, pH 7.4. They were then decalcified for 4–5 weeks in 10% ethylenediaminetetraacetic acid, dehydrated in graded alcohols, and embedded in paraffin. The sections were stained with hematoxylin and eosin (H&E) to evaluate the general morphology or with 0.1% safranin-O and fast-green to demonstrate sulfated proteoglycans (PGs). The area of safranin-O/fast-green metachromasia, indicative of PGs, and the total area of the joint cartilage in frontal sections were measured. The severity of the OA lesion was graded using Mankin's scale, which ranges from 0 to 14.8 Each data point represented the measurements of two sections from each of three individual specimens belonging to the same group disease severity group.
Real-time quantitative polymerase chain reaction
Total RNA was extracted using the Trizol reagent (Invitrogen Technologies, CA, USA) according to the manufacturer's protocol and quantified by absorbance at 260 nm. The purified RNA was reverse transcribed (RT) to cDNA using an RT-polymerase chain reaction (PCR) kit (TaKaRa, Wuhan, China) according to the provided protocol. The cDNA was either analyzed immediately or stored at -20℃. Amplification by quantitative PCR was performed using an ABI 7900 HT fast real-time PCR system (ABI, Dalian, USA), and the cDNA was quantified by using the SYBR Green I fluorescent dye method.
The final volume of the RT-PCR was 10 µL. The reaction mixture consisted of 5 µL of SYBR Premix Ex Taq, 0.2 µL each of primer (10 µmol/L), 0.2 µL of 50× ROX reference dye, and 1 µL of template, with the volume adjusted to 10 µL with distilled water. PCR cycling conditions consisted of an initial denaturing step of 10 s at 95℃, followed by 40 cycles of 5 s at 95℃ and 30 s at 60℃. A stable and reliable standard curve was established using synthesized oligonucleotides resembling cDNA fragments that were diluted in five-fold decrements. The glyceraldehyde phosphate dehydrogenase (GAPDH) gene from the same sample was used as the internal control. The GAPDH gene also served as the basis for establishing the copy number of the target gene's mRNA, thus allowing determination of the expression levels of the genes of interest and changes thereof. The specificity of each reaction was controlled by melting curve analysis. The negative PCR control contained water instead of cDNA. Real-time PCR was conducted in triplicate in three independent experiments.
Western blotting
Western blotting of the cartilage samples was carried out by using M-PER mammalian protein extraction reagent (Pierce, Rockford, USA) and protease inhibitor cocktail set III (Calbiochem, Darmstadt, Germany) plus 5 mmol EDTA/L. Twenty µg of protein was separated by SDS-PAGE on a 10% acrylamide gel. The separated proteins were transferred to a polyvinylidene difluoride membrane and subsequently blocked with 5% non-fat milk in Tris-buffer saline containing 0.05% Tween 20 (TBST). The membrane was then incubated with the corresponding antibodies (Santa Cruz, Dallas, TX, USA) in TBST overnight at 4℃, washed three times with TBST, incubated with horseradish-peroxidase-conjugated secondary antibody (1:5000) in TBST for 2 h at room temperature and visualized using the ECL kit. The relative amount of immunoreactive protein in each sample was quantitated by densitometry (AMBIS radioanalytic and visual imaging system, Ambis, USA). The densitometric data from each blot were normalized to the control condition, which was assigned a value of one for each experiment. All tests were repeated twice.
Statistical analysis
All values are expressed as mean±standard error. Statistical analysis was performed using the SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA). For the macroscopic parameters, comparisons between two groups were performed using Student's t test. Histological data were analyzed without a parametric Wilcoxon test; a p value <0.05 was considered to indicate statistical significance.
RESULTS
KL grade-depended cartilage change
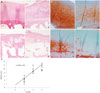
According to the radio-graphic KL grading criteria, OA patients were classified into four groups in relation to OA grading: 5 patients were categorized as grade 0, 16 as grade 2, 13 as grade 3 and 16 as grade 4. Macroscopic findings: the cartilage on condyles of healthy person were macroscopically normal. In KL grade 2 group, cartilage lesions of slight grade and size were present on condyles, Specimens from KL grade 3 and 4 groups presented a increasing severity of defect on the condyles.
H&E and safranin-O and fast green staining findings: normal cartilage had a healthful histologic appearance, Specimens from KL grade 3 and 4 group had morphologic changes, increasing cartilage fibrillation and fissures, and loss of safranin-O staining. In KL grade 0 and 2 groups, the lesions on the condyles were significantly less severe than KL grade 3 and 4 groups (Fig. 1). The cartilage degeneration was correlated with radiographic severity grading. There was positive correlation between Mankin's score and KL grades (r=0.8790, p<0.01) (Fig. 1), and Mankin's score was higher in patients with KL grade 3 and 4 than those with KL grade 0 and 2.
Correlation between HIF-2α and VEGF expression and cartilage degeneration
The expression of HIF-2α and VEGF was measured by real time-PCR and western blotting in samples acquired from the 29 patients with knee OA, 16 patients who underwent knee arthroscopy, and 5 patients with normal cartilage despite traumatic joints. HIF-2α mRNA and protein levels were lower in patients with KL grade 0 disease than in those with disease of grades 2, 3, and 4 (p<0.05) (Fig. 2) and correlated positively with the radiographic severity of knee OA (r=0.7001, p<0.05). However, the difference between KL grades 3 and 4 was not statistically significant (p>0.05).
The expression of VEGF mRNA and protein was very weak in the specimens from patients with KL grade 0 knee OA. By contrast, VEGF levels were significantly higher (Fig. 3) and correlated positively with the radiographic severity (r=0.6647, p<0.05) in patients with knee OA of higher radiographic severity (KL grades 3 and 4), including marked cartilage degeneration.
DISCUSSION
Recent studies have suggested a role for HIF-2α and VEGF in the development of OA. In the cartilage of mice with experimentally induced OA and also in human surgical specimens much higher levels of HIF-2α are expressed than in the corresponding undamaged cartilage.7
HIF-2α is encoded by the gene EPAS1 and is an extensive regulator of endochondral ossification during the development of OA. The overexpression of Epas1, either via Ad-Epas1 infection or in transgenic mice, is sufficient to trigger cartilage destruction, whereas genetic deletion of one HIF-2α allele in mice (Epas1+/-) inhibits experimentally induced OA.9 HIF-2α overexpression resulting from Ad-Epas1 infection is associated with severe cartilage destruction and a significant induction of chondrocyte apoptosis in OA cartilage.3 In our study, positive correlations were observed between HIF-2α levels, the radiographic severity of knee OA, and Mankin's score. HIF-2α expression increased with increasingly severe OA, and cartilage degeneration with increasing radiographic disease severity (KL grade). These findings are consistent with a relationship between HIF-2α levels and the severity of OA. Thus, mechanical stress may trigger OA by inducing upstream expression of NF-kB signal and HIF-2α in joint cartilage, leading to endochondral ossification by the trans-activation of COL10A1, MMP13, and VEGF.7
VEGF stimulates the mitogenic and chemotactic activities of endothelial cells and therefore angiogenesis. Although cartilage is an avascular tissue and normally produces angiogenic inhibitors,10 in pathological conditions such as OA the damaged articular cartilage is frequently covered with and invaded by highly vascular granulation tissue. Thus, VEGF is an important pro-angiogenic factor in many tissues, including diseased cartilage,11 and its levels in both plasma and synovial fluid correlate positively with the severity of knee OA.12 VEGF may destroy the framework of articular cartilage, thereby altering the tissue environment of chondrocytes and pathologically activating chondrocyte apoptosis.
There are a number of studies showing that VEGF is involved in the progression of cartilage degeneration. For example, in humans, the expression of VEGF in OA cartilage is markedly higher than in normal adult cartilage461314 and directly correlates with the Mankin score. In previous studies, we similarly showed that VEGF expression in OA cartilage is significantly higher than in normal cartilage, and that high-level expression causes the degeneration of articular cartilage by inhibiting the synthesis and expression of aggrecan and type II collagen.1516
In this study, we showed that the expression of VEGF mRNA and protein in patients with knee OA was significantly higher in those with KL grade 3 and 4 disease, characterized by severe defects in the condylar cartilage while very weak in those with KL grade 0 cartilage damage (Fig. 3). A similar correlation was found between VEGF level and the radiographic severity of the OA.
In conclusion, the significantly positive correlations between HIF-2α and VEGF levels and the radiographic severity of knee OA and the respective Mankin score suggest that these two factors can be used as biochemical markers for determining disease severity. Additional studies to elucidate the exact role of HIF-2α and VEGF in the pathogenesis of OA and to identify them as therapeutic targets are in progress.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download