Abstract
Purpose
Materials and Methods
Results
Figures and Tables
Fig. 1
Effect of Hedera helix (HH), Rhizoma coptidis (RC) extracts and the mixture of HH and RC on phenol red secretion and cough inhibition according to the dose. (A) The expectorant assay using phenol red secretion. A mixture of HH and RC extracts in a 1:1 concentration significantly increased phenol red secretion in a dose-dependent manner, and this increase was more significant than the use of the extracts individually. (B) The antitussive assay using citric acid-induced cough. A mixture of HH and RC extracts in a 1:1 concentration significantly inhibited cough in a dose-dependent manner, and this increase was also more significant than the use of the extracts individually. PC, the positive control group. *p<0.05, †p<0.01, ‡p<0.001.

Fig. 2
Effect of mixture of Hedera helix (HH) and Rhizoma coptidis (RC) extracts on phenol red secretion according to various mixing ratios. A 3:1 ratio of HH to RC showed a maximal expectorant effect. PC, the positive control group. *p<0.001.

Fig. 3
Effect of 3:1 ratio mixture of Hedera helix (HH) and Rhizoma coptidis (RC) extracts on phenol red secretion and cough inhibition according to dose. (A) In the expectorant assay using phenol red secretion, a mixture of HH and RC extracts in a 3:1 concentration showed dose-dependent increase of phenol red secretion. (B) In the antitussive assay using citric acid-induced cough, a mixture of HH and RC extracts in a 3:1 concentration significantly inhibited cough in a dose-dependent manner. PC, the positive control group. *p<0.001.

Table 1
Effect of Hedera helix (HH), Rhizoma coptidis (RC) Extracts and the Mixture of HH and RC on Phenol Red Secretion and Cough Inhibition According to Dose
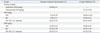
All data were compared to the negative control group and were shown as the mean±standard deviation. For the phenol red secretion experiment, each group was composed of 8 mice and 64 mice were used in total including negative control. For the cough inhibition experiment, each group was composed of 8 guinea pigs and 64 guinea pigs were used in total including negative control.
Table 2
Effect of the Mixture of Hedera helix (HH) and Rhizoma coptidis (RC) Extracts on Phenol Red Secretion According to Various Mixing Ratios

Table 3
Effect of the 3:1 Ratio Mixture of Hedera helix (HH) and Rhizoma coptidis (RC) Extracts on Phenol Red Secretion and Cough Inhibition According to Dose

All data were compared to the negative control group and were shown as the mean±standard deviation. For the phenol red secretion experiment, each group was composed of 8 mice and 40 mice were used in total including negative control. For the cough inhibition experiment, each group was composed of 8 guinea pigs and 40 guinea pigs were used in total including negative control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download