Abstract
Purpose
To compare the clinical and computed tomography (CT) appearances of liver abscesses caused by non-Klebsiella pneumoniae bacterial pathogens in elderly and nonelderly patients.
Materials and Methods
Eighty patients with confirmed non-Klebsiella pneumoniae liver abscesses (non-KPLAs) were enrolled and divided into two age groups: elderly (age ≥65 years, n=42) and nonelderly (age <65 years, n=38). Diagnosis of non-KPLA was established by pus and/or blood culture. We compared clinical presentations, outcomes, and CT characteristics of the two groups, and performed multivariate analysis for significant variables and receiver-operating-characteristic analysis to determine the cutoff value of abscess diameter for predicting non-KPLA.
Results
Elderly patients with non-KPLA were associated with a longer hospital stay (p<0.01). Regarding etiology, biliary sources had a strong association in the elderly group (p<0.01), and chronic liver diseases were related to the nonelderly group (p<0.01). Non-KPLAs (52.5%) tended to show a large, multiloculated appearance in the elderly group and were associated with bile duct dilatation (p<0.01), compared with the nonelderly group. The abscess diameter (cutoff value, 5.2 cm; area under the curve, 0.78) between the two groups was predicted. In multivariate analysis, underlying biliary tract disease [odds ratio (OR), 3.58, p<0.05], abscess diameter (OR, 2.40, p<0.05), and multiloculated abscess (OR, 1.19, p<0.01) independently predicted elderly patients with non-KPLA.
A liver abscess is potentially life-threatening and has been reported worldwide in North America, Europe, Australia, and Asia. The reported incidence ranges from 8 to 22 cases per 100000 hospital admissions, while the fatality rate ranges from 7.8% to 28.6% due to differences in species of bacterial pathogens and the underlying conditions of the infected patient, such as age, immune status, alcoholism, diabetes, and chronic lung or liver diseases.1 Currently, due to their distinct clinical outcomes, the causative bacterial organisms of liver abscesses have usually been classified as either Klebsiella pneumoniae (K. pneumoniae) or other groups of non-K. pneumoniae infections. K. pneumoniae liver abscess (KPLA) is well documented in the literature, because of its tendency to occur in diabetic patients, and it is highly associated with septic metastatic complications. With advances in diagnosis and treatment, the mortality rate of KPLA has decreased significantly in recent years. Unlike K. pneumoniae, non-K. pneumoniae pathogens, including Escherichia coli, Enterococcus species, Staphylococcus species, Streptococcus species, and Bacteroides species, are associated with a much higher mortality rate, even with an aggressive therapy, and a higher incidence of biliary disease than that of K. pneumoniae.2,3,4,5
Reviewing a few prior studies, Yang, et al.4,5,6,7 concluded that cases of non-KPLA tend to occur in geriatric populations; however, this finding was controversial because other investigators found that there was no tendency for elderly patients to have non-KPLAs.2,3,6,8 Meanwhile, the risk factors and outcomes of K. pneumoniae have been studied extensively, although that has not been the case for non-K. pneumoniae, which constitutes the greatest number of mortalities. In fact, the importance of older patients with non-KPLA is growing because of changes in the age structure of populations.5,6,7,8,9 It should be assumed that patients with non-KPLA in relatively young and older age groups have different risk profiles and imaging features. A review of the literature revealed that no previous studies had explored how the clinical features and a suspicion of non-KPLA in elderly patients differ from those of relatively young individuals. In addition, exactly how risk factors and imaging features differ between elderly and nonelderly groups with non-KPLA has not yet been established.
In order to improve knowledge of this entity, we evaluated and compared the clinical manifestations, risk factors, outcomes, and computed tomography (CT) imaging features of non-KPLA patients in different age groups.
This retrospective study was approved by the Institutional Review Board for human investigations and did not require the written informed consent of patients because confidentiality was maintained. The study was also compliant with the Health Insurance Portability and Accountability Act. We performed a retrospective computer search of our hospital database for a 7-year period (July 2003 to January 2009) using the International Classification of Diseases (9th Revision, code 572.0) to identify all patients at our institution who had a diagnosis of pyogenic liver abscess other than that caused by K. pneumoniae (i.e., non-KPLA) during their hospitalization. Pyogenic liver abscess was defined as an abscess caused by at least one bacterial origin and without amebiasis or fungal sources. Furthermore, in this study, non-KPLA had to fulfill the following criteria: 1) presence of a focal lesion in the liver parenchyma on CT images; 2) pus drained from the abscess cavity through a diagnostic, therapeutic radiological, and/or surgical drainage procedure; 3) positive bacterial culture resulting from the abscess and/or blood cultures; and 4) K. pneumoniae not isolated from the cultures.
Based on our inclusion criteria, 80 patients with clinically confirmed non-KPLA were studied, including 44 males and 36 females, with a mean age of 60 years (range, 29-95 years). In order to compare the clinical factors and imaging features of different ages, we divided our patients into two groups: elderly (age ≥65 years, n=42) and nonelderly (age <65 years, n=38).
The microbiology of the liver abscesses was defined as the organism recovered from the drained pus and/or blood culture. Etiologic pathogens from blood cultures were considered when no growth was seen in the pus culture from the liver abscess. Pus was taken for standard aerobic and anaerobic cultures, and tested for antibiotic susceptibility, along with the blood samples. Cultures resulting from samples taken at other sites of the body, including sputum and cerebrospinal fluid, were cross-matched to confirm metastatic infection, upon clinical and/or radiological evidence. After initial workup of the pus and/or blood culture, broad-spectrum antibiotics were given parenterally. The subsequent antibiotics were modified according to the results of the microbiological cultures and antibiotic susceptibility tests.
We collected data by reviewing the medical records of each patient for the following information: 1) demographic data; 2) coexisting medical conditions, including chronic obstructive pulmonary disease, chronic kidney disease (defined as estimated glomerular filtration rate <30 mL/min/1.73 m2), diabetes mellitus, biliary tract disease (defined as a stone in the bile duct, air in the biliary tree, bile duct obstruction, cholecystitis, or any previous hepatobiliary surgery), chronic liver disease (defined as alcoholism, chronic hepatitis B or C, and cirrhosis of the liver), and malignancy; 3) initial laboratory test data (including white blood cell count, hemoglobin, platelet count, C-reactive protein, total bilirubin, albumin, aspartate aminotransferase, alanine aminotransferase, and glucose); 4) clinical symptoms (including fever or right upper quadrant abdominal pain); 5) the result of the drainage procedure [successful percutaneous catheter drainage (PCD) defined as image-guided needle aspiration alone and successful PCD therapy; failed PCD defined as the patient's clinical condition that required an additional surgical procedure, such as surgical drainage or resection. Catheter blockage or dislocation was not included in this group]; 6) microbiological pathogen of the liver abscess; 7) number of days from the onset of symptoms to diagnosis; number of days that the fever subsided (body temperature of 37.5℃ or less for 2 days), number of days that leukocytosis subsided (defined as a white blood count <11000 cells/per mm3) and duration of hospitalization (defined as the number of days in hospital after the drainage procedure was performed); 8) the occurrence of drainage-related complications, including catheter dislodgement, obstruction, kinking, pneumothorax, intra-abdominal hemorrhage and bile leakage; 9) the occurrence of complications, including pleural effusion, metastatic infection, and septic shock during the same admission; and 10) mortality related to the liver abscess and its complications.
In this study, contrast-enhanced CT was performed in all patients before treatment. Patients with contraindications to contrast-enhanced CT (pregnancy, acute kidney failure, or allergy to iodinated contrast agents) were excluded. The CT protocol for right upper quadrant abdominal pain at our institution was planned as follows. Unenhanced CT scans are routinely performed without any oral administration. Intravenous contrast (Omnipaque; GE Healthcare, Norway) was administered by a power injector using a contrast concentration of 350 mg/mL, delivered at a rate of 2.5-3.0 mL/s for a volume of 90 mL. The scan delay for the arterial phase images was defined with bolus tracking, with a circular region of interest positioned at the level of the abdominal aorta; a predefined enhancement threshold level of 120 Hounsfield units (HU) was set to trigger the data acquisition. The portal venous phase was scanned 40 s after initiation of the arterial phase CT scan. A scanning range from the hepatic dome of the lower lungs to the iliac crest was used. With CT, images were routinely acquired at a beam collimation of 0.5 mm and were viewed at a slice thickness of 5 mm.
Two experienced abdominal radiologists retrospectively reviewed the CT images and developed a consensus opinion. They were aware that the patients had a liver abscess but were blinded to the patient's clinical condition. During the analysis of the CT features, cases from the elderly and nonelderly group were randomly intermixed.
The CT characteristics of the liver abscesses were recorded in terms of the following parameters: 1) location (right, left, or both lobes of the liver); 2) margin definition (ill-defined or well-defined; ill-defined was used to describe cases where more than half of the margin was finely speculated); 3) number (one, two, three, or more); 4) diameter (defined as the diameter of the largest abscess); 5) cystic appearance (defined as more than half the abscess cavity appearing liquefied, with an attenuation value of ≤20 HU on contrast-enhanced CT); 6) a multiloculated abscess (defined as an abscess with enhancing internal septations); 7) thickening of the abscess wall (measurement of the maximum wall thickness, and thickened defined as thickness ≥2 mm); 8) enhancement of the rim (defined as more than half of the margin having a higher attenuation than the surrounding liver on contrast-enhanced CT images); 9) the presence of gas density in the abscess; 10) bile duct dilatation (defined as a clear, hypodense tubular structure accompanying a contrast-opacified portal venous branch focally in the liver); 11) thrombophlebitis and/or pylephlebitis (presence of filling defects in the hepatic vein, inferior vena cava and/or portal vein); 12) pneumobilia (presence of air density in the biliary tract).
Additionally, the radiologists also recorded the presence of any underlying biliary disease and coexisting lesions in other organs (pleural effusion, lung base, and abdominal organ metastatic infection).
Statistical analyses were performed with SPSS software (SPSS 18.0; SPSS Inc., Chicago, IL, USA), and differences were considered significant when p<0.05. A comparison of the clinical findings and CT features of the elderly and nonelderly groups was performed. For the two independent age groups in our study, we used the Pearson's chi-squared or Fisher's exact test for categorical variables, and Student's t-test for continuous variables that were expressed as mean±standard deviation. If the variable was not normal distributed, we chose Mann-Whitney U test (Wilcoxon rank-sum test) to obtain the p value. Receiver-operating-characteristic (ROC) analysis was performed to determine the cutoff value for the optimal diameter between each study group. In addition, multivariable stepwise regression using binary logistic method was performed for selecting the most significant variables and obtaining their odds ratios (ORs).
Of the 80 patients with non-KPLA, 42 (52.5%) patients were in the elderly group, and 38 (47.5%) patients were in the nonelderly group (Table 1). The mean age was 70.5 years (range, 65-95 years) in the elderly group and 51.2 years (range, 29-64 years) in the nonelderly group. No significant difference in the proportions of each sex was observed between the groups.
Underlying diseases of a biliary origin (identifiable on the CT images) were the most common in the elderly group, and there was a significantly higher prevalence thereof than in the nonelderly group (66.7% vs. 31.6%, p<0.01). Chronic liver diseases were obviously less frequent in the elderly group (14.3% vs. 42.1%, p<0.01). There were no differences between groups regarding the incidence of chronic obstructive pulmonary disease, chronic kidney disease, diabetes mellitus and malignancy. The findings of the laboratory tests showed higher serum total bilirubin in the elderly group than in the nonelderly group. This was considered to be reasonable considering that more biliary tract pathogens are associated with bile duct obstruction. There were no significant differences in the other laboratory tests at admission, except that higher total bilirubin and lower albumin levels were found in the elderly group than the nonelderly group.
There were no significant differences between the two groups with respect to symptoms including fever and right upper quadrant abdominal pain (Table 2). All patients received antibiotics and one or more drainage procedures. The rates of successful PCD in the elderly and nonelderly groups were 73.8% and 78.9%, respectively, which were not significantly different. The rates of failed PCD in the elderly and nonelderly groups were also not significantly different. Specifically, in the elderly group, 11 patients experienced failed PCD therapy, with nine of these patients requiring additional surgical procedures after PCD and 2 patients receiving a surgical drainage procedure initially.
According to our definition, drainage-related complications in our study occurred in 19, including 12 (28.5%) among the 42 elderly patients and seven (18.4%) among the 38 nonelderly patients. Catheter problems, including dislodgement, obstruction, and kinking, were the most common complication in both elderly and nonelderly patient groups (n=9, or 21.4% in elderly group, and n=4, or 10.5% in nonelderly group, p=0.19). Other drainage-related complications, including pneumothorax (n=0, or 0% in elderly group, and n=1, or 2.6% in nonelderly group, p=0.29), intra-abdominal hemorrhage (n=2, or 4.8% in elderly group, and n=1, or 2.6% in nonelderly group, p=0.62), and bile leakage (n=1, or 2.4% in elderly group, and n=1, or 2.6% in nonelderly group, p=0.94) rarely occurred. Catheter problems were corrected by exchange or revision of the drainage tube. One patient who experienced pneumothorax after the procedure in the nonelderly group underwent placement of a chest tube.
Hospital stay was longer in the elderly group (31.8±11.3 days) than the nonelderly group (17.6±5.9 days); this difference was statistically significant (p<0.01). The median durations from the onset of symptoms to diagnosis, fever subsidence, and leukocytosis subsidence were higher in the elderly group; however, these differences did not reach statistical significance. Similar results were also found for the rates of mortality and associated complications of the two groups.
The species of organisms isolated in the two groups are summarized in Table 3. The overall positive growth rate of pus cultures was 77.1% (54/70), of which 70.4% (38/54) were monomicrobial. The overall positive growth rate of blood cultures was 67.1% (47/70), of which 85.1% (40/47) were monomicrobial. In both groups, the causative microorganism was predominantly Escherichia coli in both blood and pus cultures. Enterococcus species and coagulase-negative staphylococci were the second and third most common pathogens, respectively. There was no significant difference in the distribution of microorganisms between groups.
The radiographic characteristics on CT exhibited several significant differences between the elderly and nonelderly groups (Table 4). Abscesses in the elderly group appeared significantly larger (9.1±4.0 mm vs. 5.8±3.8 mm, p<0.01) and more often had a multiloculated appearance (64.3% vs. 36.8%, p=0.03) than those in the nonelderly group (Fig. 1). The rate of bile duct dilatation was significantly greater in the elderly group than in the nonelderly group (59.5% vs. 26.3%, p<0.01). There were no differences between the two groups with respect to the margin, number, and location of the abscesses, as well as the morphological pattern of the abscess cavity, including the cystic appearance, wall thickening, rim enhancement, presence of dense gas, thrombophlebitis, pylephlebitis, and pneumobilia.
ROC analysis (Fig. 2) was performed to determine the optimal value of abscess diameter in the elderly group for predicting non-KPLA in elderly patients. The ROC curve revealed that the cutoff value was 5.2 cm. Even with overlapping appearances on CT, an abscess diameter greater than 5.2 cm was predictive of a liver abscess in the elderly group. The area under the ROC curve to predict the diameter of a liver abscess in the elderly group was 0.74.
The results of univariate and multivariate stepwise logistic regression analyses of the selected significant variables are shown in Table 5. We chose five significant variables (clinical, laboratory, and imaging findings) and used a stepwise multivariate logistic regression model to identify the most significant predictors for elderly patients (>65 years) with non-KPLA. Of note, three variables (biliary tract disease, serum bilirubin level, and CT findings of biliary tract dilatation) were thought to be indicative of a similar entity; that is, highly associated with underlying biliary pathology. Since the three variables could affect each other, we decided to only include the clinical variable "biliary tract disease" as a predictor in the multivariate analysis. In the first step of multivariable analyses, the ORs for each variable as being predictive of elderly patients with non-KPLA were 3.11 for biliary tract disease, 2.39 for multiloculated abscess, 1.20 for abscess diameter, 0.68 for albumin level, and 0.18 for liver disease. In the last step, biliary tract disease (OR, 3.58, p<0.05), multiloculated abscess (OR, 2.40, p<0.05), and abscess diameter (OR, 1.19, p<0.01) were the three most significant independent predictors.
Although previous studies have shown that non-KPLA frequently occurs in the presence of an underlying biliary disease or a predisposing medical condition,2 our study is the first to investigate the clinical features and CT appearance of non-KPLA in patients of different age groups. We showed that elderly patients with non-KPLA present with a large, multiloculated abscess on CT images, which was different from those identified in the nonelderly group. An abscess diameter of 5.2 cm was the cutoff value between the two groups. In the elderly group, bile duct dilatation was a supplementary finding of non-KPLA. Moreover, a difference was also found in the comorbidities of non-KPLA, which showed that biliary sources had a strong association in the elderly group; chronic liver diseases were frequently found in the nonelderly group.
Several previous studies found that the abscess diameter is associated with important outcomes, including more complications and longer hospitalizations, as well as an increased likelihood of surgical or radiological interventions.10,11,12 Tan, et al.10 reported that larger abscesses (diameter >5 cm) were associated with an increased hospital stay and drainage failure rate. Liao, et al.12 further concluded that an abscess diameter greater than 7.3 cm was the optimal cutoff value to predict drainage failure. However, our study does not support all of these results. We found that elderly patients had larger abscesses and were even prone to a longer hospital stay, although they did not show a higher drainage failure rate. In our institution, an active drainage strategy is performed in patients with larger liver abscesses (diameter >5 cm) by implanting more than one drainage tube with a thick diameter. By our study, we have demonstrated that an active drainage strategy could provide a better clinical outcome than the previous published studies. Although there were significant differences in terms of larger abscess diameter, multiloculated abscesses, and hospital stay between elderly and nonelderly patients, we believe that an active drainage strategy could overcome drainage failures in certain cases with larger abscess diameter and reach a comparable success rate to that in the nonelderly patients.
Multiloculated abscesses were a distinctive imaging feature of non-KPLA in the elderly patients. Multiloculated abscesses have been considered to be an important factor in determining the outcome of liver abscess treatment, from either a clinical or a radiological perspective.10,13,14 Liu, et al.15 showed that with an adequate drainage strategy, multiloculated abscesses show no differences in PCD failure rate and mortality rate, compared with a single abscess. The results of our study tend to support these findings. Nevertheless, multiloculated abscesses absolutely contribute to poorer drainage by compartmentalization of the liver abscess, reducing the effectiveness of percutaneous drainage.10,16 In our experience, successful drainage of multiloculated abscesses can be achieved by irrigating the abscesses, rotating a wire to disrupt the internal septations, or placing more than one drainage tube. Most important, the use of manipulating our drainage tubes did not come at an increased complications or mortality for our patients.
Biliary tract disease was the most common underlying disease in elderly patients with non-KPLA. Furthermore, our study showed that bile duct dilatation was a characteristic imaging feature of non-KPLA in the elderly group. One of the most important findings of our study was that 80% of patients in the elderly group with underlying biliary tract disease showed CT evidence of bile duct dilatation. This association of biliary tract abnormalities emphasizes the necessity for the evaluation of biliary tract disease in elderly patients with non-KPLA. A previous study of patients with a liver abscess who underwent endoscopic retrograde cholangiopancreatography found that 75% of patients with common bile duct dilatation had an associated biliary tract pathological finding.17 Therefore, we propose that identification of a dilated bile duct by imaging in an elderly patient with a liver abscess should prompt a more detailed evaluation of the biliary tract, and consideration should be made to investigate further using imaging studies, such as magnetic resonance cholangiography or endoscopic retrograde cholangiopancreatography.
In our study, we noticed that non-KPLA in the elderly group required a longer hospitalization, compared to that in the nonelderly group. Although researchers agree that a larger, multiloculated abscess requires a longer hospital stay to adequately achieve drainage and resolution of the abscess, longer hospitalization in the elderly patients could be affected by other confounding factors. Of the associated co-morbidities in this study, the disease most related with the episode of non-KPLA in elderly patients was the underlying biliary pathology. While the prevalence of underlying biliary tract diseases was significantly different between the two age groups, we postulated that a longer hospital study is not only governed by the patient's age and the abscess diameter/morphology, but is also related to the associated underlying biliary pathology.
Our study outlined different comorbidities in the two age groups. Previous studies have shown that non-KPLA is strongly associated with the underlying biliary disease; interestingly, our study further found that patient age was a major attribute of biliary tract pathology in the non-KPLA population. Aging has an influence on both the physiological and anatomical features of the biliary tract, causing an increased incidence of biliary tract disease.18 The spread of bacterial pathogens along the biliary tree that form an abscess at the liver parenchyma, in association with bile duct dilatation, is considered to be the pathogenic mechanism. Nevertheless, chronic liver diseases were more frequently found in the nonelderly group. In the past, liver cirrhosis was considered to be a risk factor for a liver abscess.19 Our finding is thought to be due to the high prevalence of chronic hepatitis B and C infections, with associated cirrhosis, in young adults in Taiwan.
This study has several limitations that should be mentioned. First, this study was performed retrospectively at our institution, and the number of non-KPLA patients was still relatively small. We excluded patients for whom CT was not used as a diagnostic imaging modality. However, the number of patients at our institution who received only ultrasonography or magnetic resonance imaging for liver abscess was small. Second, we used an objective cutoff point of age of 65 years to allocate patients to the elderly and nonelderly age groups. Although the purpose of our study was to find the clinical and CT differences of non-KPLA in relation to age, this choice of age cutoff may cause a potential selection bias in our patient population. Last, we studied liver abscesses caused by the non-K. pneumoniae species. The causative organisms were polymicroorganisms, and this might have caused differences between the two groups. We analyzed the microbiology of isolated pus and blood cultures, and the results showed no significant difference between the groups. Therefore, there was no microbiological bias identified in this study.
In conclusion, even with overlapping CT appearances, we found that non-KPLA in the elderly patients commonly showed a large multiloculated abscess with the diameter greater than 5.2 cm. However, metabolic syndrome has often been associated with more severe liver abnormalities, and patients with non-alcoholic fatty liver disease tend to have abnormal components of metabolic syndrome.20 A larger prospective study is required for further validation of these clinical and CT variables across institutions, and a further direct comparison of elderly and nonelderly patients with non-alcoholic fatty liver disease or metabolic syndrome is also mandatory to implement an optimal diagnostic algorithm.
Figures and Tables
Fig. 1
A 72-year-old woman with non-KPLA (caused by Enterococcus) who presented with fever and right upper quadrant pain for 7 days. An axial, contrast-enhanced CT image (A) shows a large (size: about 7.9 cm) abscess (arrows) in the dome. A coronal, contrast-enhanced reconstruction CT image (B) shows the abscess with a multiloculated appearance (arrow), which was associated with bile duct dilatation (star) and pneumobilia (arrowhead). Acute cholecystitis was found. This was the most common appearance of liver abscess in the elderly. Note also the mild bilateral pleural effusions and basal atelectasis. The patient received antibiotics and PCD drainage, and she was discharged after 25 days. KPLA, Klebsiella pneumoniae liver abscess; PCD, percutaneous catheter drainage.

Fig. 2
Receiver-operating-characteristic (ROC) analysis of the minimal diameter for abscesses in the elderly group. AUC, the area under the ROC curve.

Table 1
Demographic and Biologic Characteristics of Elderly and Nonelderly Patients with Non-KPLA
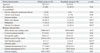
KPLA, Klebsiella pneumoniae liver abscess.
Continuous data are expressed as mean±standard deviations, and categorical data are expressed as numbers and percentages in parentheses (%).
*p<0.05.
†Since total bilirubin level is not normally distributed, Mann-Whitney U test was used for the p value. The median value of the total bilirubin level was 3.5 mg/dL in the elderly group and 1.2 mg/dL in the nonelderly group.
‡When patients fit into more than one category, they were counted in each category.
Table 2
Clinical Characteristics of the Elderly and Nonelderly Patients with Non-KPLA
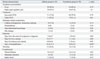
Table 3
Microbiological Isolates in Pus and Blood Cultures in Elderly and Nonelderly Patients with Non-KPLA
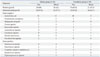
Table 4
CT Findings of Elderly and Nonelderly Patients with Non-KPLA
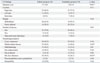
Table 5
Univariate and Multivariate Logistic Regressions for Predictors of Elderly Patients with Non-KPLA
ACKNOWLEDGEMENTS
The study was supported by the Tri-Service General Hospital Research Grant (TSGH-C101-053).
We thank the Research Office for Health Data, Department of Education and Research, Taipei City Hospital, Taiwan for their valuable contributions in data management and statistical analysis.
References
1. Foo NP, Chen KT, Lin HJ, Guo HR. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol. 2010; 105:328–335.

2. Lee NK, Kim S, Lee JW, Jeong YJ, Lee SH, Heo J, et al. CT differentiation of pyogenic liver abscesses caused by Klebsiella pneumoniae vs non-Klebsiella pneumoniae. Br J Radiol. 2011; 84:518–525.

3. Alsaif HS, Venkatesh SK, Chan DS, Archuleta S. CT appearance of pyogenic liver abscesses caused by Klebsiella pneumoniae. Radiology. 2011; 260:129–138.

4. Yang CC, Yen CH, Ho MW, Wang JH. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004; 37:176–184.
5. Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005; 100:322–331.

6. Law ST, Li KK. Older age as a poor prognostic sign in patients with pyogenic liver abscess. Int J Infect Dis. 2013; 17:e177–e184.

7. Chen SC, Lee YT, Yen CH, Lai KC, Jeng LB, Lin DB, et al. Pyogenic liver abscess in the elderly: clinical features, outcomes and prognostic factors. Age Ageing. 2009; 38:271–276.

8. Alvarez JA, González JJ, Baldonedo RF, Sanz L, Junco A, Rodrfíguez JL, et al. Pyogenic liver abscesses: a comparison of older and younger patients. HPB (Oxford). 2001; 3:201–206.

9. Kang SC, Hwang SJ. Impact of advanced age on inpatients with pyogenic liver abscess in Taiwan: a nationwide claim-based analysis. J Chin Med Assoc. 2011; 74:539–543.

10. Tan YM, Chung AY, Chow PK, Cheow PC, Wong WK, Ooi LL, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg. 2005; 241:485–490.

11. Lee CH, Leu HS, Wu TS, Su LH, Liu JW. Risk factors for spontaneous rupture of liver abscess caused by Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2005; 52:79–84.

12. Liao WI, Tsai SH, Yu CY, Huang GS, Lin YY, Hsu CW, et al. Pyogenic liver abscess treated by percutaneous catheter drainage: MDCT measurement for treatment outcome. Eur J Radiol. 2012; 81:609–615.

13. Tazawa J, Sakai Y, Maekawa S, Ishida Y, Maeda M, Marumo F, et al. Solitary and multiple pyogenic liver abscesses: characteristics of the patients and efficacy of percutaneous drainage. Am J Gastroenterol. 1997; 92:271–274.
14. Chou FF, Sheen-Chen SM, Chen YS, Chen MC. Single and multiple pyogenic liver abscesses: clinical course, etiology, and results of treatment. World J Surg. 1997; 21:384–388.

15. Liu CH, Gervais DA, Hahn PF, Arellano RS, Uppot RN, Mueller PR. Percutaneous hepatic abscess drainage: do multiple abscesses or multiloculated abscesses preclude drainage or affect outcome? J Vasc Interv Radiol. 2009; 20:1059–1065.

16. Barakate MS, Stephen MS, Waugh RC, Gallagher PJ, Solomon MJ, Storey DW, et al. Pyogenic liver abscess: a review of 10 years' experience in management. Aust N Z J Surg. 1999; 69:205–209.

17. Lam YH, Wong SK, Lee DW, Lau JY, Chan AC, Yiu RY, et al. ERCP and pyogenic liver abscess. Gastrointest Endosc. 1999; 50:340–344.

18. Walsh RM. Innovations in treating the elderly who have biliary and pancreatic disease. Clin Geriatr Med. 2006; 22:545–558.

19. Mølle I, Thulstrup AM, Jepsen P, Sørensen HT, Vilstrup H. Liver cirrhosis is risk factor for pyogenic liver abscesses. BMJ. 2001; 323:52–53.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download