Abstract
Purpose
To evaluate the effect of statin treatment on strut coverage after drug-eluting stent (DES) implantation.
Materials and Methods
In this study, 60 patients were randomly assigned to undergo sirolimus-eluting stent (SES) or biolimus-eluting stent (BES) implantation, after which patients were randomly treated with pitavastatin 2 mg or pravastatin 20 mg for 6 months. The degree of strut coverage was assessed by 6-month follow-up optical coherence tomography, which was performed in 52 DES-implanted patients.
Results
The percentages of uncovered struts were 19.4±14.7% in pitavastatin-treated patients (n=25) and 19.1±15.2% in pravastatin-treated patients (n=27; p=0.927). A lower percentage of uncovered struts was significantly correlated with a lower follow-up low-density lipoprotein (LDL) cholesterol level (r=0.486; p=0.009) and a greater decline of the LDL cholesterol level (r=-0.456; p=0.015) in SES-implanted patients, but not in BES-implanted patients. In SES-implanted patients, the percentage of uncovered struts was significantly lower among those with LDL cholesterol levels of less than 70 mg/dL after 6 months of follow-up (p=0.025), but no significant difference in this variable according to the follow-up LDL cholesterol level was noted among BES-implanted patients (p=0.971).
Neointimal coverage over stent struts emerged as an important clinical issue after it was reported that incomplete strut coverage might be associated with the occurrence of late stent thrombosis following drug-eluting stent (DES) implantation.1,2,3,4 Optical coherence tomography (OCT) is a useful tool that has excellent resolution for confirming neointimal stent strut coverage.5,6 Although several variables such as the implantation of sirolimus-eluting stents (SESs)7,8 and acute coronary syndrome9,10 were reported as risk factors for delayed strut coverage in previous OCT studies, the relationship of specific medications (i.e., statins) with strut coverage is not fully understood. Statins are the most widely used lipid-lowering agents in patients with coronary artery disease. These drugs reduce inflammation and enhance endothelial function via pleiotropic effects.11 Previous in vitro and animal studies reported that statins regulate the migration and proliferation of smooth muscle cells and control neointimal formation.12,13,14,15 However, there are no human data that assess the impact of statin treatment on strut coverage after DES implantation. Therefore, we conducted a randomized OCT study to evaluate the effect of statin treatment on DES strut coverage.
The present study consisted of subgroup analysis of a previous randomized OCT study16 and evaluated the impact of statin treatment on strut coverage. The previous OCT study was performed to compare strut coverage 6 months after Nobori biolimus-eluting stent (N-BES, Nobori®, Terumo Corporation, Tokyo, Japan; n=60) or SES (Cypher™, Cordis Corp., Miami Lakes, FL, USA; n=60) implantation. The inclusion and exclusion criteria of the OCT study were provided in the previous report.16 Written informed consent was obtained from all participants, and the Institutional Review Boards of our institute approved this study. In total, 60 patients were enrolled in this study and randomly treated with pitavastatin 2 mg or pravastatin 20 mg, beginning on the day of stent implantation. Stenting (SES or N-BES) was also randomly allocated. Thus, four groups were created as follows: pitavastatin-N-BES group (12 patients), pitavastatin-SES group (17 patients), pravastatin-N-BES group (18 patients), and pravastatin-SES group (13 patients). Post-intervention OCT examinations were performed for all patients immediately after DES implantation. Among 60 patients, 6-month follow-up angiography was not performed for three patients, the OCT catheter could not be passed through the lesion due to severe angulation in three patients, and there was poor image quality in two patients. Therefore, follow-up OCT evaluation was performed for 52 patients as follows: pitavastatin-N-BES group (10 patients), pitavastatin-SES group (15 patients), pravastatin-N-BES group (14 patients), and pravastatin-SES group (13 patients) (Fig. 1). Blood samples to evaluate lipid profiles were obtained at the time of stent implantation and follow-up angiography. All patients were clinically followed up 1, 3, and 6 months after stent implantation.
All patients received at least 75 mg of aspirin and a loading dose of 300 mg of clopidogrel at least 12 h pre-intervention. Unfractionated heparin was administered to maintain the activated clotting time at >250 s. All percutaneous coronary interventions were performed according to current standard techniques. Post-intervention, dual antiplatelet therapy with aspirin 100 mg and clopidogrel 75 mg daily was prescribed for 12 months.
Quantitative coronary angiographic analysis was performed before and after stent implantation as well as at follow-up, using an offline quantitative coronary angiographic system (CASS system, Pie Medical Instruments, Maastricht, the Netherlands) in an independent core laboratory (Cardiovascular Research Center, Seoul, Korea). Using the guiding catheter for magnification and calibration, reference vessel diameters and the minimal luminal diameter were measured from diastolic frames in a single, matched view showing the smallest minimal luminal diameter. Late loss was defined as the difference between the post-procedure and follow-up minimal luminal diameters.
Immediately and 6 months after the intervention, OCT of the target lesion was performed using a frequency-domain OCT system (C7-XR OCT imaging system, LightLab Imaging, Inc., St. Jude Medical, St. Paul, MN, USA). For this study, OCT cross-sectional images were generated at a rotational speed of 100 frames per second while the fiber was withdrawn at a speed of 20 mm/s within the stationary imaging sheath. All OCT images were analyzed at a core laboratory (Cardiovascular Research Center, Seoul, Korea) by analysts who were blinded to patient and procedural information.
Cross-sectional OCT images were analyzed at 0.2-mm longitudinal intervals. Stent and luminal cross-sectional areas (CSAs) were measured, and the neointimal hyperplasia (NIH) CSA was calculated as the stent area minus the luminal CSA. NIH thickness was measured as the distance between the endoluminal surface of the neointima and the strut.8 An uncovered strut was categorized as an NIH thickness=0 µm.8 A malapposed strut was defined as a strut that had detached from the vessel wall by ≥130 µm (N-BES) or ≥160 µm (SES).17,18 The percentage of uncovered or malapposed struts was calculated as the ratio of uncovered or malapposed struts to total struts in all OCT cross-sections. Intrastent thrombi were defined as irregular masses protruding into the lumen by more than 250 µm at the thickest point.19
Statistical analysis was performed using Statistical Analysis System software (v. 9.1.3., SAS Institute, Cary, NC, USA) and R version 2.15.1 (R Development Core Team, Vienna, Austria, http://www.R-project.org). Categorical data were presented as numbers (%) and compared using the chi-square or Fisher's exact test. Continuous data were presented as the mean±SD and compared using a paired t-test, Student's t-test, or the Mann-Whitney U test. To avoid problems of sample size inflation and correlated data, only patients with one target lesion were included in the study. Cross-section analysis or strut-level analysis may not be straightforward due to the congregation of struts within each lesion in an interindividual manner. For this analysis, we performed multilevel regression model analysis. Specifically, the patient and lesion data were incorporated as random effect components using the lme4 package with R (http://cran.r-project.org/web/packages/lme4/index.html).20 Pearson's correlation analysis was performed to evaluate the relationship between low-density lipoprotein (LDL) cholesterol levels and the percentage of uncovered struts. Values of p<0.05 denoted statistical significance.
Baseline clinical and angiographic characteristics were similar between the pitavastatin-treated groups and pravastatin-treated groups (Table 1). Significant reductions of LDL cholesterol levels were observed at the 6-month follow-up time point (reduction: 24 mg/dL in the pitavastatin-treated group, p<0.001; 21 mg/dL in the pravastatin-treated group, p= 0.003). Follow-up LDL cholesterols level less than 70 mg/dL were achieved in 10 patients (34.5%) in the pitavastatin-treated group and 5 patients (16.1%) in the pravastatin-treated group (p=0.101). OCT findings were also similar between pitavastatin-treated patients and pravastatin-treated patients (Table 2). The percentages of uncovered struts at the 6-month follow-up OCT were 19.4±14.7% in pitavastain-treated patients (n=25) and 19.1±15.2% in pravastain-treated patients (n=27) (p=0.927); conversely, the values were 23.3±16.6% in SES-implanted patients (n=28) and 14.5±10.9% in BES-implanted patients (n=24) (p=0.026).
In all of 52 patients, follow-up cholesterol levels of less than 70 mg/dL were associated with smaller percentages of uncovered struts (less than 70 mg/dL vs. 70 mg/dL or more; 12.5±12.2% vs. 21.5±15.1%; p=0.058). Although the percentage of uncovered struts among SES-implanted patients was significantly lower in patients with follow-up LDL cholesterol levels of less than 70 mg/dL (less than 70 mg/dL vs. 70 mg/dL or more; 10.1±12.4% vs. 26.9±15.6%, respectively; p=0.025), there were no significant differences in the percentage of uncovered struts among BES-implanted patients between those with follow-up LDL cholesterol levels of less than 70 mg/dL (14.6±12.7%) and those with levels exceeding 70 mg/dL (14.4±10.5%; p=0.971). Fig. 2 presents the relationship of follow-up LDL cholesterol levels with strut coverage according to DES type. The percentage of uncovered struts was significantly correlated with follow-up LDL cholesterol levels (r=0.486; p=0.009) in SES-implanted patients but not in BES-implanted patients. Fig. 3 presents the relationship of the degree of LDL cholesterol reduction with strut coverage according to DES type. The percentage of uncovered struts was also significantly correlated with the degree of LDL cholesterol reduction (r=-0.456; p=0.015) in SES-implanted patients but not in BES-implanted patients.
Previous studies identified several factors that were associated with delayed strut coverage, such as SES implantation,7,8 acute coronary syndrome,9,10 the time interval between DES implantation and follow-up OCT,21 baseline high-sensitivity C-reactive protein levels,22 the stent diameter,23 diabetes mellitus,23 and American Heart Association/American College of Cardiology type B2/C lesions.23 Although several studies revealed that statins have beneficial effects on both mature endothelial cells and endothelial progenitor cells,24,25,26,27 there are no human data associating the use of statins with an acceleration of strut coverage after DES implantation. Wang, et al.14 demonstrated that atorvastatin pretreatment could accelerate both neointimal coverage and reendothelialization after SES implantation in a minipig model. Another animal study using a wire-mediated vascular injury model in mice reported that fluvastatin has protective effects against impaired reendothelialization in sirolimus-treated arteries.24 The mechanism by which statins protect against delayed vascular healing is linked to their ability to modulate smooth muscle cell proliferation and migration and increase circulating endothelial progenitor cell counts.14,24 Experimental animal studies reported that each statin has different effects according to its water solubility.28,29
In this study, strut coverage was compared between pita-vastatin-treated lesions and pravastatin-treated lesions, and we hypothesized that different statins may differentially affect OCT-based strut coverage in DES-implanted lesions. Compared to pravastatin (a hydrophilic statin), pitavastatin (a fully synthetic lipophilic statin) has powerful efficacy comparable with that of atorvastatin and rosuvastatin.30,31 In the present study, a significant difference in strut coverage was not observed between the pitavastatin-treated groups and pravastatin-treated groups; however, greater DES strut coverage was significantly related with lower follow-up LDL cholesterol levels and greater reductions of LDL cholesterol levels in SES-implanted lesions, but not in BES-implanted lesions.
These findings suggest that reducing LDL cholesterol levels alone has a role in strut coverage after first-generation DES implantation. However, as mentioned previously, the primary mechanism by which statins modify the vascular healing process is considered to involve a pleotropic effect rather than a direct LDL cholesterol-lowering effect. Therefore, this discrepancy might be explained by the fact that a lower LDL cholesterol level is one indicator of the intensity of the pleotropic effect of statins in the vascular healing process. Additionally, the relationship between follow-up LDL cholesterol levels and strut coverage was not observed for next-generation DESs (i.e., BESs). A possible explanation for this finding is that the vascular healing response to statins or lower LDL cholesterol levels could be different according to the type of DES. Compared to SESs, BESs have several different characteristics, including a bioresorbable polymer carrier (poly-lactic acid), as well as coating only on the abluminal stent surface to allow the direct release of lipophilic biolimus into the vessel wall.32,33 Differences in the polymer, drug, or drug-eluting period between BESs and SESs may influence the degree of strut coverage according to the LDL cholesterol-lowering effects of statins.
The present study has some limitations. First, although our study was designed as a randomized trial, the study population included a relatively small number of patients. We feel that a future study with a larger sample size would be needed to confirm our findings. However, our main findings could provide a clue, suggesting the possible relationship of lowering LDL cholesterol or use of statin with the vascular healing process. Second, all control groups received statin therapy. Therefore, we could not evaluate the effect of statin therapy on strut coverage by making comparisons with patients who did not receive statin treatment. However, a control group with no statin treatment would not be ethically justified in current clinical practice for DES-implanted patients with coronary artery disease.
In conclusion, this randomized study revealed a protective effect of statins against delayed strut coverage in SES-implanted patients who achieved lower follow-up LDL cholesterol levels (especially less than 70 mg/dL). This vascular healing effect of lower LDL cholesterol levels induced by statins could be different according to the type of DES implanted.
Figures and Tables
Fig. 2
The relationships of the percentage of uncovered struts with follow-up low-density lipoprotein (LDL) cholesterol levels are presented. Black and red dots represent biolimus-eluting stents and sirolimus-eluting stents, respectively.
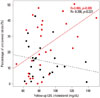
Fig. 3
The relationships of the percentage of uncovered struts with the degree of low-density lipoprotein (LDL) cholesterol level reduction are presented. Black and red dots represent biolimus-eluting stents and sirolimus-eluting stents, respectively.

Table 1
Baseline Clinical and Angiographic Characteristics of the Pitavastatin-Treated Groups and Pravastatin-Treated Groups

Table 2
Optical Coherence Tomography (OCT) Findings of the Pitavastatin-Treated Groups and Pravastatin-Treated Groups
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (nos. A085012 and A102064), a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (no. A085136), and the Cardiovascular Research Center, Seoul, Korea.
References
1. Farb A, Burke AP, Kolodgie FD, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003; 108:1701–1706.

2. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007; 115:2435–2441.

3. Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, Ikejima H, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012; 5:12–20.

4. Won H, Shin DH, Kim BK, Mintz GS, Kim JS, Ko YG, et al. Optical coherence tomography derived cut-off value of uncovered stent struts to predict adverse clinical outcomes after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2013; 29:1255–1263.

5. Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010; 31:401–415.

6. Lee SY, Hong MK. Stent evaluation with optical coherence tomography. Yonsei Med J. 2013; 54:1075–1083.

7. Kim JS, Jang IK, Kim JS, Kim TH, Takano M, Kume T, et al. Optical coherence tomography evaluation of zotarolimus-eluting stents at 9-month follow-up: comparison with sirolimus-eluting stents. Heart. 2009; 95:1907–1912.

8. Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K, et al. Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am J Cardiol. 2007; 99:1033–1038.

9. Kim JS, Fan C, Choi D, Jang IK, Lee JM, Kim TH, et al. Different patterns of neointimal coverage between acute coronary syndrome and stable angina after various types of drug-eluting stents implantation; 9-month follow-up optical coherence tomography study. Int J Cardiol. 2011; 146:341–346.

10. Kubo T, Imanishi T, Kitabata H, Kuroi A, Ueno S, Yamano T, et al. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable angina pectoris: a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2008; 1:475–484.

11. Mihos CG, Salas MJ, Santana O. The pleiotropic effects of the hydroxy-methyl-glutaryl-CoA reductase inhibitors in cardiovascular disease: a comprehensive review. Cardiol Rev. 2010; 18:298–304.

12. Indolfi C, Cioppa A, Stabile E, Di Lorenzo E, Esposito G, Pisani A, et al. Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferation in vitro and neointimal formation in vivo after vascular injury. J Am Coll Cardiol. 2000; 35:214–221.

13. Bellosta S, Bernini F, Ferri N, Quarato P, Canavesi M, Arnaboldi L, et al. Direct vascular effects of HMG-CoA reductase inhibitors. Atherosclerosis. 1998; 137:Suppl. S101–S109.

14. Wang TJ, Yang YJ, Xu B, Zhang Q, Jin C, Tang Y, et al. Atorvastatin accelerates both neointimal coverage and re-endothelialization after sirolimus-eluting stent implantation in a porcine model: new findings from optical coherence tomography and pathology. Circ J. 2012; 76:2561–2571.
15. Jaschke B, Michaelis C, Milz S, Vogeser M, Mund T, Hengst L, et al. Local statin therapy differentially interferes with smooth muscle and endothelial cell proliferation and reduces neointima on a drug-eluting stent platform. Cardiovasc Res. 2005; 68:483–492.

16. Kim BK, Ha J, Mintz GS, Kim JS, Shin DH, Ko YG, et al. Randomised comparison of strut coverage between Nobori biolimus-eluting and sirolimus-eluting stents: an optical coherence tomography analysis. EuroIntervention. 2014; 9:1389–1397.

17. Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Di Mario C. The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomography. Int J Cardiol. 2009; 134:180–188.

18. Davlouros PA, Mavronasiou E, Xanthopoulou I, Karantalis V, Tsigkas G, Hahalis G, et al. An optical coherence tomography study of two new generation stents with biodegradable polymer carrier, eluting paclitaxel vs. biolimus-A9. Int J Cardiol. 2012; 157:341–346.

19. Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006; 97:1713–1717.

20. Doran H, Bates D, Bliese P, Dowling M. Estimating the multilevel Rasch model: with the lme4 package. J Stat Softw. 2007; 20:1–18.
21. Kim BK, Kim JS, Oh C, Ko YG, Choi D, Jang Y, et al. Major determinants for the uncovered stent struts on optical coherence tomography after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2012; 28:705–714.

22. Kim BK, Kim JS, Oh C, Ko YG, Choi D, Jang Y, et al. Impact of preprocedural high-sensitivity C-reactive protein levels on uncovered stent struts: an optical coherence tomography study after drug-eluting stent implantation. Clin Cardiol. 2011; 34:97–101.

23. Ishigami K, Uemura S, Morikawa Y, Soeda T, Okayama S, Nishida T, et al. Long-term follow-up of neointimal coverage of sirolimus-eluting stents--evaluation with optical coherence tomography. Circ J. 2009; 73:2300–2307.

24. Fukuda D, Enomoto S, Shirakawa I, Nagai R, Sata M. Fluvastatin accelerates re-endothelialization impaired by local sirolimus treatment. Eur J Pharmacol. 2009; 612:87–92.

25. Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol. 2003; 23:729–736.

26. Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002; 22:1567–1572.

27. Walter DH, Zeiher AM, Dimmeler S. Effects of statins on endothelium and their contribution to neovascularization by mobilization of endothelial progenitor cells. Coron Artery Dis. 2004; 15:235–242.

28. Sakamoto T, Kojima S, Ogawa H, Shimomura H, Kimura K, Ogata Y, et al. Usefulness of hydrophilic vs lipophilic statins after acute myocardial infarction: subanalysis of MUSASHI-AMI. Circ J. 2007; 71:1348–1353.

29. Satoh K, Ichihara K. Lipophilic HMG-CoA reductase inhibitors increase myocardial stunning in dogs. J Cardiovasc Pharmacol. 2000; 35:256–262.

30. Saku K, Zhang B, Noda K. PATROL Trial Investigators. Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL): the PATROL trial. Circ J. 2011; 75:1493–1505.

31. Hayashi T, Yokote K, Saito Y, Iguchi A. Pitavastatin: efficacy and safety in intensive lipid lowering. Expert Opin Pharmacother. 2007; 8:2315–2327.

32. Chevalier B, Silber S, Park SJ, Garcia E, Schuler G, Suryapranata H, et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial--Phase 2. Circ Cardiovasc Interv. 2009; 2:188–195.

33. Ostojic M, Sagic D, Beleslin B, Jung R, Perisic Z, Jagic N, et al. First clinical comparison of Nobori -Biolimus A9 eluting stents with Cypher- Sirolimus eluting stents: Nobori Core nine months angiographic and one year clinical outcomes. EuroIntervention. 2008; 3:574–579.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download