Abstract
Purpose
Coronary artery disease (CAD) shares several risk factors with abdominal aortic aneurysm (AAA). We evaluated the prevalence during transthoracic echocardiography (TTE) and risk factors of AAA in patients with CAD.
Materials and Methods
A total of 1300 CAD patients were screened from August 2009 to May 2010, and measurement of abdominal aorta size was feasible in 920 patients (71%) at the end of routine TTE. An AAA was defined as having a maximal diameter of ≥30 mm.
Results
Of the 920 patients, 22 (2.4% of the study population) were diagnosed with AAA; of these AAA patients, 86% were male, and 82% were over 65 years-old. Abdominal aortic size was weakly correlated with aortic root diameter (r=0.22, p<0.01). Although the proportions of male gender, hypertension, and dyslipidemia were higher in AAA patients, such differences were not statistically significant. Advanced age [odds ratio (OR)=1.07; 95% confidence interval (CI): 1.01-1.12; p<0.01], smoking (OR=3.44; 95% CI: 1.18-10.04; p=0.02), and peripheral arterial disease (OR=5.88; 95% CI: 1.38-25.05; p=0.01) were found to be associated with AAA.
Conclusion
Although prevalence of AAA is very low in the Asian population, the prevalence of AAA in Asian CAD patients is higher than the general population. Therefore, opportunistic examination of the abdominal aorta during routine TTE could be effective, especially for male CAD patients over 65 years with a history of smoking or peripheral arterial disease.
An abdominal aortic aneurysm (AAA) is defined as an aorta size of more than 30 mm or regional dilation of the abdominal aorta by more than 50%. AAA usually remains asymptomatic unless it ruptures, and in cases of rupture, operative mortality rate often exceeds 50%.1 However, if patients undergo elective surgery for AAA, hospital mortality rate is greatly reduced to <5%.2 Therefore, early diagnosis of AAA is crucial, and a screening of AAA is recommended especially for a high risk population.
The prevalence of AAA has been reported in Western countries as 1.3-8.9% in men and 1.0-2.2% in women.3,4,5 On the contrary, the prevalence of AAA in the Asian population has not been studied thoroughly. Previously, we have reported that the prevalence of AAA in the Korean population is 0.5%, found by the routine screening for AAA during clinical transthoracic echocardiography (TTE).6
AAA is considered a manifestation of atherosclerosis,7 and coronary artery disease (CAD) may be associated with a higher prevalence of AAA than the normal population8,9 Also, AAA and CAD share several risk factors such as male gender, advanced age, hypertension, smoking, peripheral arterial disease, and hypercholesterolemia.10,11 A high incidence of adverse cardiovascular events such as cardiovascular death, myocardial infarction, and stroke were observed in patients with AAA and CAD.12 However, there have been few studies regarding the prevalence of AAA in Asians with CAD.
TTE is usually performed on CAD patients for a clinical purpose; thus, echocardiographic protocol can be modified for use as a screening method for AAA. The purpose of this study was to investigate the prevalence of AAA during TTE and the associated risk factors of AAA with CAD patients in Korea.
From August 2009 to May 2010 at Samsung Medical Center, screening for AAA during TTE was prospectively performed in 1300 consecutive patients who had been diagnosed with significant CAD. Of these patients, reliable measurements of abdominal aorta size were available in 920 patients (71%). Intestinal gas, obesity or body habitus, and surgical wounds after coronary artery bypass grafting (CABG) were the reasons for inadequate visualization of the abdominal aorta in 380 patients (29%). Among these patients, 99 (10.8%) had their TTE done after CABG; echocardiographic windows of the abdominal aorta could not be acquired due to surgical wounds from CABG. The analysis was based on the 920 patients (677 men and 243 women) from whom reliable aorta size measurements were available (Fig. 1). As a retrospective study, there was no need to obtain informed consent from each patient.
Significant coronary artery stenosis was defined as a narrowing of ≥50% in diameter of the epicardial segment of the coronary artery, as observed through coronary angiography (CAG). All clinical data and laboratory data were collected using an electrical recording system in a prospective manner. This study was approved by the Institutional Review Board of our hospital.
TTE images were acquired by experienced sonographers, interpreted by echocardiographers, and prospectively recorded in an electrical reporting system. No instructions on food or fluid intake were given prior to the examination. Routine examinations, which included 2-dimension, M-mode, and Doppler techniques, were performed, and measurements were made according to the guidelines of the American Society of Echocardiography. At the end of the examination, the abdominal aorta was visualized with the patient in supine position, as previously described,6 using a Vivid E9 (GE Healthcare, Horten, Norway) or SC2000 ultrasound (Siemens Medical Solutions, Mountain View, CA, USA) and a 3.7 Hz transducer. First, a longitudinal image of the abdominal aorta was visualized with the transducer marker pointing toward the patient's feet. The transducer was then rotated 90° counterclockwise and adjusted to scan the orthogonal plane perpendicular to the central line of the abdominal aorta. The infrarenal abdominal aorta was visualized below the origin of the renal artery and then traced distally as far as possible. The maximum short-axial diameter of the abdominal aorta was measured at the antero-posterior plane of the abdominal aorta because that has been shown to be more reproducible than transverse diameter.13 The average examination time required for the evaluation of the abdominal aorta was about 2 minutes. An abdominal aorta greater than 30 mm was recognized as an AAA.
Hypertension was defined as systolic blood pressure of 140 mm Hg or higher, diastolic blood pressure of 90 mm Hg or higher, or use of oral antihypertensive medication.14 Smoking history was classified as either current smoker or past smoker. Patients who smoked at least 1 cigarette/day were defined as current smokers, and patients who had quit smoking for at least 1 year were defined as past smokers. Dyslipidemia was defined as total cholesterol level >240 mg/dL, low-density lipoprotein cholesterol level >130 mg/dL, high-density lipoprotein cholesterol level <40 mg/dL for men or <50 mg/dL for women, triglyceride level >200 mg/dL, or use of antihyperlipidemic medication.15 Diabetes mellitus was defined as fasting plasma glucose level ≥126 mg/dL, random plasma glucose level ≥200 mg/dL in patients with classic hyperglycemic symptoms, use of oral hypoglycemic drugs or insulin, or life style modifications for the treatment of known diabetes.16 Peripheral arterial disease (PAD) was defined as Rutherford claudication stage ≥3, history of treatment for chronic limb ischemia, or ankle brachial pressure index value ≤0.9.17
Continuous variables were described as mean±standard deviation. Categorical variables were expressed as numbers and percentages (%). The chi-square test was used for the comparison of categorical variables, and the independent sample t-test was applied for the comparison of continuous variables. A p-value less than 0.05 was considered statistically significant. Statistical analysis was performed with IBM SPSS Statistics (Version 19.0, IBM SPSS Inc., Chicago, IL, USA).
The baseline demographic variables of the study population are shown in Table 1. The mean age of the study population was 64±10 years. The mean abdominal aorta size was 19.1±5.2 mm. AAA was diagnosed in 22 patients of 920 CAD patients (2.4% of the study population), 19 of who were male (86% of AAA patients). Among them, 9 patients had already been diagnosed with AAA by a different imaging study and 13 patients (1.4%) were newly diagnosed with AAA from the TTE study.
All 3 women with AAA were over 70 years old. The mean age of AAA patients was 69±6 years, and 82% of AAA patients were older than 65 years. Considering only the male patients aged over 65 years, the 4.9% of patients (15 of 306) were found to have an AAA. The prevalence of AAA in male patients over 65 years with a smoking history was 7.1% (11 of 154).
Patients with AAA were older than those without AAA (AAA: 69±6; non-AAA: 64±10 years; p<0.01) and more often male than female, but without statistical significance (male: 86%; female: 73%; p=0.16). The mean AAA size was 42.7±11.7 mm. Among the 13 newly diagnosed patients, AAA was confirmed by abdominal computed tomography for only 3 patients as the other patients were found to have smaller AAAs. One patient (7.6%) had an aortic diameter greater than 50 mm and underwent an elective surgical open repair of AAA. The other two patients underwent endovascular aneurysm repair in order to treat associated thoracic aortic aneurysms. There were no serious complications after AAA repair.
We compared the cardiovascular risk factors (Table 2) and echocardiographic findings (Table 3) of CAD patients with and without AAA. Although the proportion of male gender and the prevalence of hypertension and dyslipidemia were higher in AAA, they were not statistically significant. Diabetes mellitus was inversely associated with AAA and was statistically insignificant. Multiple logistic regression analysis showed advanced age [odds ratio (OR)=1.07; 95% confidence interval (CI): 1.01-1.12; p<0.01], smoking (OR= 3.44; 95% CI: 1.18-10.04; p=0.02), and PAD (OR=5.88; 95% CI: 1.38-25.05; p=0.01) as the independent risk factors for AAA.
Aortic root (sinuses of valsalva) size measured by TTE was greater in patients with AAA than in those without AAA (AAA: 35 mm; non-AAA: 33 mm; p<0.01). Aortic root size was weakly correlated with abdominal aortic size in Fig. 2 (r=0.22; p<0.01). After adjustment for age (r=0.22; p<0.01), gender (r=0.24; p<0.01), and smoking status (r=0.23; p<0.01), weak correlation was observed between abdominal aortic size and aortic root size. Other echocardiographic variables were not significantly different between groups (Table 3).
We investigated the prevalence of AAA in CAD patients through a modified screening protocol using TTE. We found that the prevalence of those newly diagnosed with AAA by screening using TTE was 2.4% in CAD patients who underwent CAG in Korea. The prevalence tended to increase with age and for males. For example, among males over 65 years old with CAD, the prevalence of AAA reached 4.9%. Several previous studies of Caucasian CAD patients have reported varying rates of AAA prevalence ranging from 6.9% to 14% (Supplementary Table 1, only online).8,9,18,19,20,21 As most of these studies are composed of males older than 60 years who had severe CAD and underwent CABG, prevalence in these studies may be much higher than those in subjects without CAD. Regarding the disposition of AAA in CAD patients, a recent study with 35418 individuals in Sweden22 showed a higher incidence of AAA in northern Sweden, corresponding well with reported CAD patterns and suggesting that the presence of CAD is linked to risk of AAA.
However, there is little data about the prevalence of AAA in CAD patients in the Asian population. One previous study composed of Chinese patients with severe CAD and awaiting elective CABG showed a much lower prevalence of 1.8% compared to the prevalence reported with Caucasians (Supplementary Table 1, only online).21 The present study shows results similar to the study involving Chinese patients, which supports the claim that the prevalence of AAA is found to be lower in Asians with CAD than in Caucasians with CAD. In this study, we enrolled patients with any significant CAD diagnosed by CAG. Thus, our study population includes rather mild forms of CAD than other reports. Although the prevalence of AAA in Koreans with CAD is low (2.4% of the study population) when compared with Western reports, this prevalence in CAD patients may be nearly five times higher than the general Korean population (0.5%) in our previous report.6 To the best of our knowledge, this is the first study to focus on AAA prevalence in Korean CAD patients.
Several previous studies demonstrated that smoking is strongly associated with the risk of AAA.10,23,24,25 Smoking has been suggested as indicator for AAA screening even in women of age >65 years.26 Svensjö, et al.27 reported a low prevalence of AAA among 65-year-old Swedish men and explained that decreasing numbers of smokers compared to previous generations is associated with a change in the epidemiology of the disease. Smoking not only promotes atherosclerosis but may also block the active site of α1-antitrypsin, which could promote the destruction of the aortic wall by proteolytic enzymes in an additional nonatheroscleritic pathway.25,28 Other studies reported increased plasma levels of matrix metalloproteinases-1, -2, and -9 in patients with AAA,29,30,31 and this observation may be related to the effects of smoking on elastase activity and elastin degradation in the vessel wall media.32
TTE is almost routinely performed in CAD patients for evaluating left ventricular (LV) systolic function, detecting regional LV wall motion abnormalities and complications, and assessing prognosis. Our study revealed that the feasibility of visualization of abdominal aortic size was considerably high (71%). Moreover, evaluation of the abdominal aortic size during TTE is not time consuming or laborious, nor is there additional cost. Considering the finding that CAD patients present with a higher prevalence of AAA and are more frequently examined with TTE, patients with CAD could be considered as a selective group for AAA screening, which can take place during TTE to save costs. That effect would be even more augmented in 65 year old male CAD patients with a history of smoking or peripheral arterial disease.
This study has several limitations. First, the prevalence of AAA in CAD in our study population was low; slightly undermining the statistical reliability of the risk factors of AAA. Second, we included the postoperative patients who underwent CABG, and these patients' echocardiographic windows were frequently poor, which indicates that the feasibility of AAA screening using TTE may be low for such patients.
In conclusion, the prevalence of AAA in CAD was low but higher than the general population, as observed in opportunistic examinations of abdominal aorta during routine TTE. The prevalence tended to increase with older males. Of traditional risk factors, advanced age, smoking, and PAD were statistically significant risk factors of AAA in CAD patients. Therefore, opportunistic examination of the abdominal aorta during routine TTE could be effective, especially for male CAD patients over 65 years with a history of smoking or PAD.
Figures and Tables
Fig. 1
Study population. CAD, coronary artery disease; AAA, abdominal aortic aneurysm; TTE, transthoracic echocardiography; CABG, coronary artery bypass grafting.

Table 1
Demographic Variables of the Study Population

Table 2
Comparison of Cardiovascular Risk Factors in CAD Patients with and without AAA

Table 3
Echocardiographic Characteristics of Patients with and without AAA
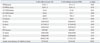
AAA, abdominal aortic aneurysm; CAD, coronary artery disease; IVSd, interventricular septal wall thickness at diastole; LVPWd, left ventricular posterior wall thickness at diastole; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; LV EF, left ventricular ejection fraction; LA, left atrium; E, early diastolic mitral inflow velocity; A, late diastolic mitral inflow velocity; DT, deceleration time of E velocity; E', early diastolic septal mitral annular velocity; A', late diastolic septal mitral annular velocity.
References
1. Ingoldby CJ, Wujanto R, Mitchell JE. Impact of vascular surgery on community mortality from ruptured aortic aneurysms. Br J Surg. 1986; 73:551–553.

2. Graham M, Chan A. Ultrasound screening for clinically occult abdominal aortic aneurysm. CMAJ. 1988; 138:627–629.
4. Lederle FA, Johnson GR, Wilson SE. Aneurysm Detection and Management Veterans Affairs Cooperative Study. Abdominal aortic aneurysm in women. Abdominal aortic aneurysm in women. J Vasc Surg. 2001; 34:122–126.

5. Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000; 160:1425–1430.

6. Oh SH, Chang SA, Jang SY, Park SJ, Choi JO, Lee SC, et al. Routine screening for abdominal aortic aneurysm during clinical transthoracic echocardiography in a Korean population. Echocardiography. 2010; 27:1182–1187.

7. Reed D, Reed C, Stemmermann G, Hayashi T. Are aortic aneurysms caused by atherosclerosis. Circulation. 1992; 85:205–211.

8. Bergersen L, Kiernan MS, McFarlane G, Case TD, Ricci MA. Prevalence of abdominal aortic aneurysms in patients undergoing coronary artery bypass. Ann Vasc Surg. 1998; 12:101–105.

9. Madaric J, Vulev I, Bartunek J, Mistrik A, Verhamme K, De Bruyne B, et al. Frequency of abdominal aortic aneurysm in patients >60 years of age with coronary artery disease. Am J Cardiol. 2005; 96:1214–1216.

10. Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994-2001. Circulation. 2009; 119:2202–2208.
11. Cornuz J, Sidoti Pinto C, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health. 2004; 14:343–349.

12. Miura T, Soga Y, Doijiri T, Aihara H, Yokoi H, Iwabuchi M, et al. Prevalence and clinical outcome of polyvascular atherosclerotic disease in patients undergoing coronary intervention. Circ J. 2013; 77:89–95.

13. Becker F, Baud JM. Groupe de Travail Ad Hoc. [Screening for abdominal aortic aneurysm and surveillance of small abdominal aortic aneurysms, rationale and recommendations of the French Society for Vascular Medicine. Final document]. J Mal Vasc. 2006; 31:260–276.
14. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–2572.

15. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
16. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013; 36:Suppl 1. S67–S74.
17. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)--summary of recommendations. J Vasc Interv Radiol. 2006; 17:1383–1397.
18. Monney P, Hayoz D, Tinguely F, Cornuz J, Haesler E, Mueller XM, et al. High prevalence of unsuspected abdominal aortic aneurysms in patients hospitalised for surgical coronary revascularisation. Eur J Cardiothorac Surg. 2004; 25:65–68.

19. Dupont A, Elkalioubie A, Juthier F, Tagzirt M, Vincentelli A, Le Tourneau T, et al. Frequency of abdominal aortic aneurysm in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2010; 105:1545–1548.

20. Long A, Bui HT, Barbe C, Henni AH, Journet J, Metz D, et al. Prevalence of abdominal aortic aneurysm and large infrarenal aorta in patients with acute coronary syndrome and proven coronary stenosis: a prospective monocenter study. Ann Vasc Surg. 2010; 24:602–608.

21. Poon JT, Cheng SW, Wong JS, Ting AC. Prevalence of abdominal aortic aneurysm in Chinese patients with severe coronary artery disease. ANZ J Surg. 2010; 80:630–633.

22. Hultgren R, Forsberg J, Alfredsson L, Swedenborg J, Leander K. Regional variation in the incidence of abdominal aortic aneurysm in Sweden. Br J Surg. 2012; 99:647–653.

23. Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am J Epidemiol. 2001; 154:236–244.

24. Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006; 26:2605–2613.
25. Lee AJ, Fowkes FG, Carson MN, Leng GC, Allan PL. Smoking, atherosclerosis and risk of abdominal aortic aneurysm. Eur Heart J. 1997; 18:671–676.

26. Derubertis BG, Trocciola SM, Ryer EJ, Pieracci FM, McKinsey JF, Faries PL, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg. 2007; 46:630–635.

27. Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011; 124:1118–1123.

28. Cohen JR, Sarfati I, Ratner L, Tilson D. Alpha 1-antitrypsin phenotypes in patients with abdominal aortic aneurysms. J Surg Res. 1990; 49:319–321.
29. Kowalewski R, Sobolewski K, Małkowski A, Wolan´ska M, Gacko M. Evaluation of enzymes involved in proteoglycan degradation in the wall of abdominal aortic aneurysms. J Vasc Res. 2006; 43:95–100.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download